Assembly of the Tripartite and RNA Condensates of the Respiratory Syncytial Virus Factory Proteins In Vitro: Role of the Transcription Antiterminator M2-1
- PMID: 37376628
- PMCID: PMC10303025
- DOI: 10.3390/v15061329
Assembly of the Tripartite and RNA Condensates of the Respiratory Syncytial Virus Factory Proteins In Vitro: Role of the Transcription Antiterminator M2-1
Abstract
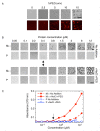
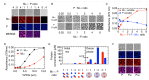
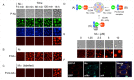
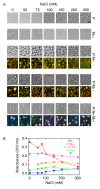
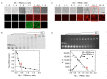
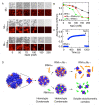
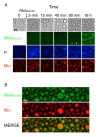
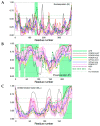
A wide variety of viruses replicate in liquid-like viral factories. Non-segmented negative stranded RNA viruses share a nucleoprotein (N) and a phosphoprotein (P) that together emerge as the main drivers of liquid-liquid phase separation. The respiratory syncytial virus includes the transcription antiterminator M2-1, which binds RNA and maximizes RNA transcriptase processivity. We recapitulate the assembly mechanism of condensates of the three proteins and the role played by RNA. M2-1 displays a strong propensity for condensation by itself and with RNA through the formation of electrostatically driven protein-RNA coacervates based on the amphiphilic behavior of M2-1 and finely tuned by stoichiometry. M2-1 incorporates into tripartite condensates with N and P, modulating their size through an interplay with P, where M2-1 is both client and modulator. RNA is incorporated into the tripartite condensates adopting a heterogeneous distribution, reminiscent of the M2-1-RNA IBAG granules within the viral factories. Ionic strength dependence indicates that M2-1 behaves differently in the protein phase as opposed to the protein-RNA phase, in line with the subcompartmentalization observed in viral factories. This work dissects the biochemical grounds for the formation and fate of the RSV condensates in vitro and provides clues to interrogate the mechanism under the highly complex infection context.
Keywords: LLPS; M2-1; Mononegavirales; antiterminator; condensates; nucleocapsid; phosphoprotein; respiratory syncytial virus; viral factories.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fields B.N. In: Fields Virology. 6th ed. Knipe D.M., Howley P.M., editors. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

