The Aqueous Leaf Extract of the Medicinal Herb Costus speciosus Suppresses Influenza A H1N1 Viral Activity under In Vitro and In Vivo Conditions
- PMID: 37376674
- PMCID: PMC10303568
- DOI: 10.3390/v15061375
The Aqueous Leaf Extract of the Medicinal Herb Costus speciosus Suppresses Influenza A H1N1 Viral Activity under In Vitro and In Vivo Conditions
Abstract
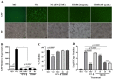
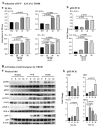
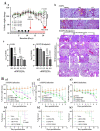
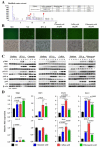
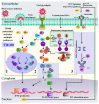
This study investigated the antiviral activity of aqueous leaf extract of Costus speciosus (TB100) against influenza A. Pretreatment of TB100 in RAW264.7 cells enhanced antiviral activity in an assay using the green fluorescence-expressing influenza A/Puerto Rico/8/1934 (H1N1) virus. The fifty percent effective concentration (EC50) and fifty percent cytotoxic concentration (CC50) were determined to be 15.19 ± 0.61 and 117.12 ± 18.31 µg/mL, respectively, for RAW264.7 cells. Based on fluorescent microscopy, green fluorescence protein (GFP) expression and viral copy number reduction confirmed that TB100 inhibited viral replication in murine RAW264.7 and human A549 and HEp2 cells. In vitro pretreatment with TB100 induced the phosphorylation of transcriptional activators TBK1, IRF3, STAT1, IKB-α, and p65 associated with interferon pathways, indicating the activation of antiviral defenses. The safety and protective efficacy of TB100 were assessed in BALB/c mice as an oral treatment and the results confirmed that it was safe and effective against influenza A/Puerto Rico/8/1934 (H1N1), A/Philippines/2/2008 (H3N2), and A/Chicken/Korea/116/2004 (H9N2). High-performance liquid chromatography of aqueous extracts led to the identification of cinnamic, caffeic, and chlorogenic acids as potential chemicals for antiviral responses. Further confirmatory studies using these acids revealed that each of them confers significant antiviral effects against influenza when used as pretreatment and enhances the antiviral response in a time-dependent manner. These findings suggest that TB100 has the potential to be developed into an antiviral agent that is effective against seasonal influenza.
Keywords: A (H3N2); A(H9N2); Costus speciosus; TB100; antiviral effect; influenza A (H1N1); leaf extract; transcriptional activator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

