m6A Regulates the Stability of Cellular Transcripts Required for Efficient KSHV Lytic Replication
- PMID: 37376680
- PMCID: PMC10303434
- DOI: 10.3390/v15061381
m6A Regulates the Stability of Cellular Transcripts Required for Efficient KSHV Lytic Replication
Abstract
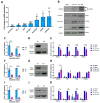
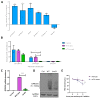
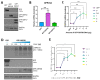
The epitranscriptomic modification N6-methyladenosine (m6A) is a ubiquitous feature of the mammalian transcriptome. It modulates mRNA fate and dynamics to exert regulatory control over numerous cellular processes and disease pathways, including viral infection. Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation from the latent phase leads to the redistribution of m6A topology upon both viral and cellular mRNAs within infected cells. Here we investigate the role of m6A in cellular transcripts upregulated during KSHV lytic replication. Our results show that m6A is crucial for the stability of the GPRC5A mRNA, whose expression is induced by the KSHV latent-lytic switch master regulator, the replication and transcription activator (RTA) protein. Moreover, we demonstrate that GPRC5A is essential for efficient KSHV lytic replication by directly regulating NFκB signalling. Overall, this work highlights the central importance of m6A in modulating cellular gene expression to influence viral infection.
Keywords: GPCR5A; KSHV; RNA modification; cell signalling; lytic replication; m6A methylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

