An Engineered Adeno-Associated Virus Capsid Mediates Efficient Transduction of Pericytes and Smooth Muscle Cells of the Brain Vasculature
- PMID: 37376759
- PMCID: PMC10457656
- DOI: 10.1089/hum.2022.211
An Engineered Adeno-Associated Virus Capsid Mediates Efficient Transduction of Pericytes and Smooth Muscle Cells of the Brain Vasculature
Erratum in
-
Correction to: An Engineered Adeno-Associated Virus Capsid Mediates Efficient Transduction of Pericytes and Smooth Muscle Cells of the Brain Vasculature, by Ramirez et al. Hum Gene Ther 2023;34(15-16):682-696; doi: 10.1089/hum.2022.211.Hum Gene Ther. 2023 Dec;34(23-24):1273. doi: 10.1089/hum.2022.211.correx. Epub 2023 Oct 30. Hum Gene Ther. 2023. PMID: 37902985 Free PMC article. No abstract available.
Abstract
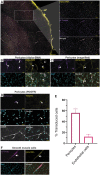
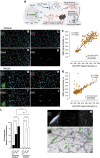
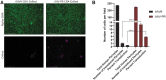
Neurodegeneration and cerebrovascular disease share an underlying microvascular dysfunction that may be remedied by selective transgene delivery. To date, limited options exist in which cellular components of the brain vasculature can be effectively targeted by viral vector therapeutics. In this study, we characterize the first engineered adeno-associated virus (AAV) capsid mediating high transduction of cerebral vascular pericytes and smooth muscle cells (SMCs). We performed two rounds of in vivo selection with an AAV capsid scaffold displaying a heptamer peptide library to isolate capsids that traffic to the brain after intravenous delivery. One identified capsid, termed AAV-PR, demonstrated high transduction of the brain vasculature, in contrast to the parental capsid, AAV9, which transduces mainly neurons and astrocytes. Further analysis using tissue clearing, volumetric rendering, and colocalization revealed that AAV-PR enabled high transduction of cerebral pericytes located on small-caliber vessels and SMCs in the larger arterioles and penetrating pial arteries. Analysis of tissues in the periphery indicated that AAV-PR also transduced SMCs in large vessels associated with the systemic vasculature. AAV-PR was also able to transduce primary human brain pericytes with higher efficiency than AAV9. Compared with previously published AAV capsids tropisms, AAV-PR represents the first capsid to allow for effective transduction of brain pericytes and SMCs and offers the possibility of genetically modulating these cell types in the context of neurodegeneration and other neurological diseases.
Keywords: adeno-associated virus vector; pericytes; smooth muscle cells.
Conflict of interest statement
C.A.M. has a financial interest in Sphere Gene Therapeutics, Inc., Chameleon Biosciences, Inc., and Skylark Bio, Inc., companies developing gene therapy platforms. C.A.M.'s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies. C.A.M., P.L.M., E.H., and K.S.H. have a filed patent application surrounding the AAV-PR capsid.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

