Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice
- PMID: 37377116
- PMCID: PMC10393077
- DOI: 10.1093/eurheartj/ehad381
Sirtuin 2 deficiency aggravates ageing-induced vascular remodelling in humans and mice
Abstract
Aims: The mechanisms underlying ageing-induced vascular remodelling remain unclear. This study investigates the role and underlying mechanisms of the cytoplasmic deacetylase sirtuin 2 (SIRT2) in ageing-induced vascular remodelling.
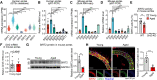
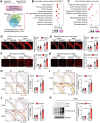
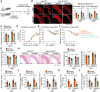
Methods and results: Transcriptome and quantitative real-time PCR data were used to analyse sirtuin expression. Young and old wild-type and Sirt2 knockout mice were used to explore vascular function and pathological remodelling. RNA-seq, histochemical staining, and biochemical assays were used to evaluate the effects of Sirt2 knockout on the vascular transcriptome and pathological remodelling and explore the underlying biochemical mechanisms. Among the sirtuins, SIRT2 had the highest levels in human and mouse aortas. Sirtuin 2 activity was reduced in aged aortas, and loss of SIRT2 accelerated vascular ageing. In old mice, SIRT2 deficiency aggravated ageing-induced arterial stiffness and constriction-relaxation dysfunction, accompanied by aortic remodelling (thickened vascular medial layers, breakage of elastin fibres, collagen deposition, and inflammation). Transcriptome and biochemical analyses revealed that the ageing-controlling protein p66Shc and metabolism of mitochondrial reactive oxygen species (mROS) contributed to SIRT2 function in vascular ageing. Sirtuin 2 repressed p66Shc activation and mROS production by deacetylating p66Shc at lysine 81. Elimination of reactive oxygen species by MnTBAP repressed the SIRT2 deficiency-mediated aggravation of vascular remodelling and dysfunction in angiotensin II-challenged and aged mice. The SIRT2 coexpression module in aortas was reduced with ageing across species and was a significant predictor of age-related aortic diseases in humans.
Conclusion: The deacetylase SIRT2 is a response to ageing that delays vascular ageing, and the cytoplasm-mitochondria axis (SIRT2-p66Shc-mROS) is important for vascular ageing. Therefore, SIRT2 may serve as a potential therapeutic target for vascular rejuvenation.
Keywords: Ageing; Arterial stiffness; SIRT2; Vascular remodelling; mROS; p66Shc.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





Comment in
-
Sirtuin 2 in vascular ageing: the forsaken child?Eur Heart J. 2023 Aug 1;44(29):2760-2762. doi: 10.1093/eurheartj/ehad366. Eur Heart J. 2023. PMID: 37377081 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

