Binding of Nitriles and Isonitriles to V(III) and Mo(III) Complexes: Ligand vs Metal Controlled Mechanism
- PMID: 37377337
- PMCID: PMC10336974
- DOI: 10.1021/acs.inorgchem.3c00595
Binding of Nitriles and Isonitriles to V(III) and Mo(III) Complexes: Ligand vs Metal Controlled Mechanism
Abstract
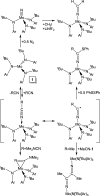
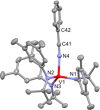
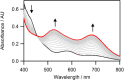
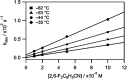
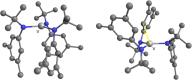
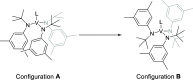
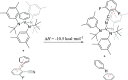
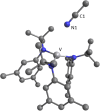
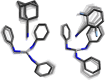
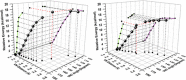
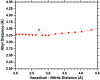
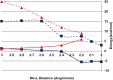
The synthesis and structures of nitrile complexes of V(N[tBu]Ar)3, 2 (Ar = 3,5-Me2C6H3), are described. Thermochemical and kinetic data for their formation were determined by variable temperature Fourier transform infrared (FTIR), calorimetry, and stopped-flow techniques. The extent of back-bonding from metal to coordinated nitrile indicates that electron donation from the metal to the nitrile plays a less prominent role for 2 than for the related complex Mo(N[tBu]Ar)3, 1. Kinetic studies reveal similar rate constants for nitrile binding to 2, but the activation parameters depend critically on the nature of R in RCN. Activation enthalpies range from 2.9 to 7.2 kcal·mol-1, and activation entropies from -9 to -28 cal·mol-1·K-1 in an opposing manner. Density functional theory (DFT) calculations provide a plausible explanation supporting the formation of a π-stacking interaction between a pendant arene of the metal anilide of 2 and the arene substituent on the incoming nitrile in favorable cases. Data for ligand binding to 1 do not exhibit this range of activation parameters and are clustered in a small area centered at ΔH‡ = 5.0 kcal·mol-1 and ΔS‡ = -26 cal·mol-1·K-1. Computational studies are in agreement with the experimental data and indicate a stronger dependence on electronic factors associated with the change in spin state upon ligand binding to 1.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


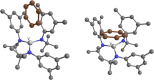

References
-
- Tobe M. L.; Burgess J.. Inorganic Reaction Mechanisms; Addison Wesley Longman Inc.: New York, 1999.
- Ašperger S.Chemical Kinetics and Inorganic Reaction Mechanisms; Springer: New York, 2003.
-
- Stephan G. C.; Peters G.; Lehnert N.; Habeck C. M.; Nather C.; Tuczek F. Bonding, activation, and protonation of dinitrogen on a molybdenum pentaphosphine complex — Comparison to trans-bis(dinitrogen) and -nitrile – dinitrogen complexes with tetraphosphine coordination. Can. J. Chem. 2005, 83, 385–402. 10.1139/v05-015. - DOI
- Germain M. E.; Temprado M.; Castonguay A.; Kryatova O. P.; Rybak-Akimova E. V.; Curley J. J.; Mendiratta A.; Tsai Y.-C.; Cummins C. C.; Prabhakar R.; McDonough J. E.; Hoff C. D. Coordination-mode control of bound nitrile radical complex reactivity: intercepting end-on nitrile–Mo(III) radicals at low temperature. J. Am. Chem. Soc. 2009, 131, 15412–15423. 10.1021/ja905849a. - DOI - PubMed
- Achord P.; Fujita E.; Muckerman J. T.; Scott B.; Fortman G. C.; Temprado M.; Cai X.; Captain B.; Isrow D.; Weir J. J.; McDonough J. E.; Hoff C. D. Experimental and computational studies of binding of dinitrogen, nitriles, azides, diazoalkanes, pyridine, and pyrazines to M(PR3)2(CO)3 (M = Mo, W; R = Me, iPr). Inorg. Chem. 2009, 48, 7891–904. 10.1021/ic900764e. - DOI - PubMed
- Hidai M.; Mizobe Y. Research inspired by the chemistry of nitrogenase — Novel metal complexes and their reactivity toward dinitrogen, nitriles, and alkynes. Can. J. Chem. 2005, 83, 358–374. 10.1139/v05-016. - DOI
-
- Burford R. J.; Fryzuk M. D. Examining the relationship between coordination mode and reactivity of dinitrogen. Nat. Rev. Chem. 2017, 1, 002610.1038/s41570-017-0026. - DOI
- Kim S.; Loose F.; Chirik P. J. Beyond ammonia: nitrogen-element bond forming reactions with coordinated dinitrogen. Chem. Rev. 2020, 120, 5637–5681. 10.1021/acs.chemrev.9b00705. - DOI - PubMed
- Singh D.; Buratto W. R.; Torres J. F.; Murray L. J. Activation of dinitrogen by polynuclear metal complexes. Chem. Rev. 2020, 120, 5517–5581. 10.1021/acs.chemrev.0c00042. - DOI - PMC - PubMed
- Sorsche D.; Miehlich M. E.; Searles K.; Gouget G.; Zolnhofer E. M.; Fortier S.; Chen C.-H.; Gau M.; Carroll P. J.; Murray C. B.; Caulton K. G.; Khusniyarov M. M.; Meyer K.; Mindiola D. J. Unusual dinitrogen binding and electron storage in dinuclear iron complexes. J. Am. Chem. Soc. 2020, 142, 8147–8159. 10.1021/jacs.0c01488. - DOI - PubMed
- del Horno E.; Jover J.; Mena M.; Pérez-Redondo A.; Yélamos C. Dinitrogen binding at a trititanium chloride complex and its conversion to ammonia under ambient conditions. Angew. Chem. 2022, 134, e20220454410.1002/ange.202204544. - DOI - PMC - PubMed
-
- Bokach N. A.; Kukushkin V. Y. Coordination chemistry of dialkylcyanamides: Binding properties, synthesis of metal complexes, and ligand reactivity. Coord. Chem. Rev. 2013, 257, 2293–2316. 10.1016/j.ccr.2013.03.002. - DOI
- Michelin R. A.; Mozzon M.; Bertani R. Reactions of transition metal-coordinated nitriles. Coord. Chem. Rev. 1996, 147, 299–338. 10.1016/0010-8545(94)01128-1. - DOI
-
- Laplaza C. E.; Odom A. L.; Davis W. M.; Cummins C. C.; Protasiewicz J. D. Cleavage of the nitrous oxide NN bond by a tris(amido)molybdenum(III) complex. J. Am. Chem. Soc. 1995, 117, 4999–5000. 10.1021/ja00122a033. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

