Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring
- PMID: 37377608
- PMCID: PMC10266428
- DOI: 10.1158/2767-9764.CRC-23-0144
Mitochondria Transfer from Mesenchymal Stem Cells Confers Chemoresistance to Glioblastoma Stem Cells through Metabolic Rewiring
Abstract
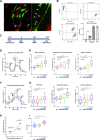
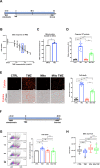
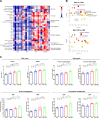
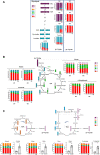
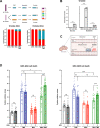
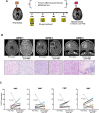
Glioblastomas (GBM) are heterogeneous tumors with high metabolic plasticity. Their poor prognosis is linked to the presence of glioblastoma stem cells (GSC), which support resistance to therapy, notably to temozolomide (TMZ). Mesenchymal stem cells (MSC) recruitment to GBM contributes to GSC chemoresistance, by mechanisms still poorly understood. Here, we provide evidence that MSCs transfer mitochondria to GSCs through tunneling nanotubes, which enhances GSCs resistance to TMZ. More precisely, our metabolomics analyses reveal that MSC mitochondria induce GSCs metabolic reprograming, with a nutrient shift from glucose to glutamine, a rewiring of the tricarboxylic acid cycle from glutaminolysis to reductive carboxylation and increase in orotate turnover as well as in pyrimidine and purine synthesis. Metabolomics analysis of GBM patient tissues at relapse after TMZ treatment documents increased concentrations of AMP, CMP, GMP, and UMP nucleotides and thus corroborate our in vitro analyses. Finally, we provide a mechanism whereby mitochondrial transfer from MSCs to GSCs contributes to GBM resistance to TMZ therapy, by demonstrating that inhibition of orotate production by Brequinar (BRQ) restores TMZ sensitivity in GSCs with acquired mitochondria. Altogether, these results identify a mechanism for GBM resistance to TMZ and reveal a metabolic dependency of chemoresistant GBM following the acquisition of exogenous mitochondria, which opens therapeutic perspectives based on synthetic lethality between TMZ and BRQ.
Significance: Mitochondria acquired from MSCs enhance the chemoresistance of GBMs. The discovery that they also generate metabolic vulnerability in GSCs paves the way for novel therapeutic approaches.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. - PubMed
-
- Horbinski C, Nabors LB, Portnow J, Baehring J, Bhatia A, Bloch O, et al. . NCCN guidelines® insights: central nervous system cancers, version 2.2022. J Natl Compr Canc Netw 2023;21:12–20. - PubMed
-
- MacLeod G, Bozek DA, Rajakulendran N, Monteiro V, Ahmadi M, Steinhart Z, et al. . Genome-wide CRISPR-cas9 screens expose genetic vulnerabilities and mechanisms of temozolomide sensitivity in glioblastoma stem cells. Cell Rep 2019;27:971–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

