Iron homeostasis imbalance and ferroptosis in brain diseases
- PMID: 37377861
- PMCID: PMC10292684
- DOI: 10.1002/mco2.298
Iron homeostasis imbalance and ferroptosis in brain diseases
Abstract
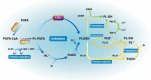
Brain iron homeostasis is maintained through the normal function of blood-brain barrier and iron regulation at the systemic and cellular levels, which is fundamental to normal brain function. Excess iron can catalyze the generation of free radicals through Fenton reactions due to its dual redox state, thus causing oxidative stress. Numerous evidence has indicated brain diseases, especially stroke and neurodegenerative diseases, are closely related to the mechanism of iron homeostasis imbalance in the brain. For one thing, brain diseases promote brain iron accumulation. For another, iron accumulation amplifies damage to the nervous system and exacerbates patients' outcomes. In addition, iron accumulation triggers ferroptosis, a newly discovered iron-dependent type of programmed cell death, which is closely related to neurodegeneration and has received wide attention in recent years. In this context, we outline the mechanism of a normal brain iron metabolism and focus on the current mechanism of the iron homeostasis imbalance in stroke, Alzheimer's disease, and Parkinson's disease. Meanwhile, we also discuss the mechanism of ferroptosis and simultaneously enumerate the newly discovered drugs for iron chelators and ferroptosis inhibitors.
Keywords: ferroptosis; iron chelators; iron homeostasis; neurodegenerative diseases; stroke.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Gell DA. Structure and function of haemoglobins. Blood Cells Mol Dis. 2018;70:13‐42. - PubMed
-
- Fitzpatrick PF. The aromatic amino acid hydroxylases: structures, catalysis, and regulation of phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase. Arch Biochem Biophys. 2023;735:109518. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
