Synthesis, ADMT prediction, and in vitro and in silico α-glucosidase inhibition evaluations of new quinoline-quinazolinone-thioacetamides
- PMID: 37377867
- PMCID: PMC10291282
- DOI: 10.1039/d3ra01790g
Synthesis, ADMT prediction, and in vitro and in silico α-glucosidase inhibition evaluations of new quinoline-quinazolinone-thioacetamides
Abstract
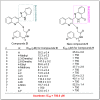
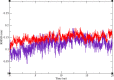
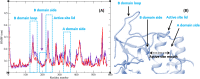
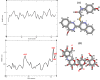
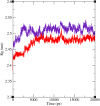
In this work, a new series of quinoline-quinazolinone-thioacetamide derivatives 9a-p were designed using a combination of effective pharmacophores of the potent α-glucosidase inhibitors. These compounds were synthesized by simple chemical reactions and evaluated for their anti-α-glucosidase activity. Among the tested compounds, compounds 9a, 9f, 9g, 9j, 9k, and 9m demonstrated significant inhibition effects in comparison to the positive control acarbose. Particularly, compound 9g with inhibitory activity around 83-fold more than acarbose exhibited the best anti-α-glucosidase activity. Compound 9g showed a competitive type of inhibition in the kinetic study, and the molecular simulation studies demonstrated that this compound with a favorable binding energy occupied the active site of α-glucosidase. Furthermore, in silico ADMET studies of the most potent compounds 9g, 9a, and 9f were performed to predict their drug-likeness, pharmacokinetic, and toxicity properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

