Emergence of functionally aberrant and subsequent reduction of neuromuscular connectivity and improved motor performance after cervical spinal cord injury in Rhesus
- PMID: 37378049
- PMCID: PMC10291623
- DOI: 10.3389/fresc.2023.1205456
Emergence of functionally aberrant and subsequent reduction of neuromuscular connectivity and improved motor performance after cervical spinal cord injury in Rhesus
Abstract
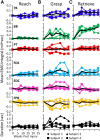
Introduction: The paralysis that occurs after a spinal cord injury, particularly during the early stages of post-lesion recovery (∼6 weeks), appears to be attributable to the inability to activate motor pools well beyond their motor threshold. In the later stages of recovery, however, the inability to perform a motor task effectively can be attributed to abnormal activation patterns among motor pools, resulting in poor coordination.
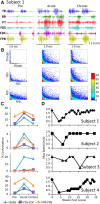
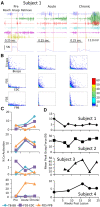
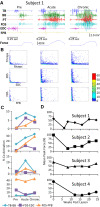
Method: We have tested this hypothesis on four adult male Rhesus monkeys (Macaca mulatta), ages 6-10 years, by recording the EMG activity levels and patterns of multiple proximal and distal muscles controlling the upper limb of the Rhesus when performing three tasks requiring different levels of skill before and up to 24 weeks after a lateral hemisection at C7. During the recovery period the animals were provided routine daily care, including access to a large exercise cage (5' × 7' × 10') and tested every 3-4 weeks for each of the three motor tasks.
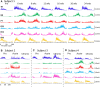
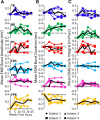
Results: At approximately 6-8 weeks the animals were able to begin to step on a treadmill, perform a spring-loaded task with the upper limb, and reaching, grasping, and eating a grape placed on a vertical stick. The predominant changes that occurred, beginning at ∼6-8 weeks of the recovery of these tasks was an elevated level of activation of most motor pools well beyond the pre-lesion level.
Discussion: As the chronic phase progressed there was a slight reduction in the EMG burst amplitudes of some muscles and less incidence of co-contraction of agonists and antagonists, probably contributing to an improved ability to selectively activate motor pools in a more effective temporal pattern. Relative to pre-lesion, however, the EMG patterns even at the initial stages of recovery of successfully performing the different motor tasks, the level of activity of most muscle remained higher. Perhaps the most important concept that emerges from these data is the large combinations of adaptive strategies in the relative level of recruitment and the timing of the peak levels of activation of different motor pools can progressively provide different stages to regain a motor skill.
Keywords: EMG; motor performance; muscle activity patterns; spinal cord injury; spinal plasticity.
© 2023 Wai, Zdunowski, Zhong, Nielson, Ferguson, Strand, Moseanko, Hawbecker, Nout-Lomas, Rosenzweig, Beattie, Bresnahan, Tuszynski, Roy and Edgerton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta).Brain. 2005 Oct;128(Pt 10):2338-58. doi: 10.1093/brain/awh604. Epub 2005 Jul 27. Brain. 2005. PMID: 16049043
-
EMG for assessing the recovery of voluntary movement after acute spinal cord injury in man.Clin Neurophysiol. 2004 Aug;115(8):1748-59. doi: 10.1016/j.clinph.2004.03.002. Clin Neurophysiol. 2004. PMID: 15261853
-
Changes in ipsilesional hand motor function differ after unilateral injury to frontal versus frontoparietal cortices in Macaca mulatta.Exp Brain Res. 2020 Jan;238(1):205-220. doi: 10.1007/s00221-019-05690-0. Epub 2019 Dec 13. Exp Brain Res. 2020. PMID: 31834452
-
Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury.Exp Neurol. 2021 Jun;340:113672. doi: 10.1016/j.expneurol.2021.113672. Epub 2021 Feb 27. Exp Neurol. 2021. PMID: 33652030 Review.
-
Sensorimotor adaptations to microgravity in humans.J Exp Biol. 2001 Sep;204(Pt 18):3217-24. doi: 10.1242/jeb.204.18.3217. J Exp Biol. 2001. PMID: 11581337 Review.
Cited by
-
Comparative efficacy of robotic exoskeleton and conventional gait training in patients with spinal cord injury: a meta-analysis of randomized controlled trials.J Neuroeng Rehabil. 2025 May 29;22(1):121. doi: 10.1186/s12984-025-01649-1. J Neuroeng Rehabil. 2025. PMID: 40442684 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

