Health-related quality of life and clinical complexity of a real-life cohort of patients with advanced HR+/HER2- breast cancer treated with CDK4/6 inhibitors and endocrine therapy
- PMID: 37378079
- PMCID: PMC10291968
- DOI: 10.7573/dic.2023-1-7
Health-related quality of life and clinical complexity of a real-life cohort of patients with advanced HR+/HER2- breast cancer treated with CDK4/6 inhibitors and endocrine therapy
Abstract
Background: Advanced breast cancer (ABC) is characterized by multidimensional clinical complexity that is usually not considered in randomized clinical trials. In the present real-life study, we investigated the link between clinical complexity and quality of life of patients with HR+/HER2- ABC treated with CDK4/6 inhibitors.
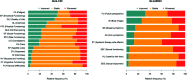
Methods: We evaluated multimorbidity burden assessed with the Cumulative Illness Rating Scale (CIRS), polypharmacy and patient-reported outcomes (PROs). PROs were assessed at baseline (T0), after 3 months of therapy (T1), and at disease progression (T2) using EORTC QLC-C30 and QLQ-BR23 questionnaires. Baseline PROs and changes between T0 and T1 were evaluated amongst patients with different multimorbidity burden (CIRS <5 and ≥5) and polypharmacy (<2 or ≥2 drugs).
Results: From January 2018 to January 2022, we enrolled 54 patients (median age 66 years, IQR 59-74). The median CIRS score was 5 (IQR 2-7), whilst the median number of drugs taken by patients was 2 (IQR 0-4). No changes in QLQ-C30 final scoring between T0 and T1 were observed in the overall cohort (p=0.8944). At T2, QLQ-C30 global score deteriorated with respect to baseline (p=0.0089). At baseline, patients with CIRS ≥5 had worse constipation than patients without comorbidities (p<0.05) and a lower trend in the median QLQ-C30 global score. Patients on ≥2 drugs had lower QLQ-C30 final scores and worse insomnia and constipation (p<0.05). No change in QLQ-C30 final score from T0 to T1 was observed (p>0.05).
Conclusion: Multimorbidity and polypharmacy increase the clinical complexity of patients with ABC and may affect baseline PROs. The safety profile of CDK4/6 inhibitors seems to be maintained in this population. Further studies are needed to assess clinical complexity in patients with ABC.This article is part of the Tackling clinical complexity in breast cancer Special Issue: https://www.drugsincontext.com/special_issues/tackling-clinical-complexity-in-breast-cancer/.
Keywords: CDK4/6 inhibitors; breast cancer; clinical complexity; comorbidities; polypharmacy; quality of life; real-life population.
Copyright © 2023 Tagliaferri B, Mollica L, Palumbo R, Leli C, Malovini A, Terzaghi M, Quaquarini E, Teragni C, Maccarone S, Premoli A, Sottotetti F.
Conflict of interest statement
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this article. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/05/dic.2023-1-7-COI.pdf
Figures

References
-
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

