Defining the Roles of Pyruvate Oxidation, TCA Cycle, and Mannitol Metabolism in Methicillin-Resistant Staphylococcus aureus Catheter-Associated Urinary Tract Infection
- PMID: 37378538
- PMCID: PMC10433999
- DOI: 10.1128/spectrum.05365-22
Defining the Roles of Pyruvate Oxidation, TCA Cycle, and Mannitol Metabolism in Methicillin-Resistant Staphylococcus aureus Catheter-Associated Urinary Tract Infection
Abstract
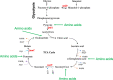
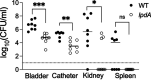
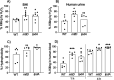
Methicillin-resistant Staphylococcus aureus (MRSA) is an important cause of complicated urinary tract infection (UTI) associated with the use of indwelling urinary catheters. Previous reports have revealed host and pathogen effectors critical for MRSA uropathogenesis. Here, we sought to determine the significance of specific metabolic pathways during MRSA UTI. First, we identified four mutants from the Nebraska transposon mutant library in the MRSA JE2 background that grew normally in rich medium but displayed significantly reduced growth in pooled human urine (HU). This prompted us to transduce the uropathogenic MRSA 1369 strain with the transposon mutants in sucD and fumC (tricarboxylic acid [TCA] cycle), mtlD (mannitol metabolism), and lpdA (pyruvate oxidation). Notably, sucD, fumC, and mtlD were also significantly upregulated in the MRSA 1369 strain upon exposure to HU. Compared to the WT, the MRSA 1369 lpdA mutant was significantly defective for (i) growth in HU, and (ii) colonization of the urinary tract and dissemination to the kidneys and the spleen in the mouse model of catheter-associated UTI (CAUTI), which may be attributed to its increased membrane hydrophobicity and higher susceptibility to killing by human blood. In contrast to their counterparts in the JE2 background, the sucD, fumC, and mtlD mutants in the MRSA 1369 background grew normally in HU; however, they displayed significant fitness defects in the CAUTI mouse model. Overall, identification of novel metabolic pathways important for the urinary fitness and survival of MRSA can be used for the development of novel therapeutics. IMPORTANCE While Staphylococcus aureus has historically not been considered a uropathogen, S. aureus urinary tract infection (UTI) is clinically significant in certain patient populations, including those with chronic indwelling urinary catheters. Moreover, most S. aureus strains causing catheter-associated UTI (CAUTI) are methicillin-resistant S. aureus (MRSA). MRSA is difficult to treat due to limited treatment options and the potential to deteriorate into life-threatening bacteremia, urosepsis, and shock. In this study, we found that pathways involved in pyruvate oxidation, TCA cycle, and mannitol metabolism are important for MRSA fitness and survival in the urinary tract. Improved understanding of the metabolic needs of MRSA in the urinary tract may help us develop novel inhibitors of MRSA metabolism that can be used to treat MRSA-CAUTI more effectively.
Keywords: Krebs cycle; MRSA; TCA cycle; mannitol metabolism; metabolism; methicillin-resistant Staphylococcus aureus; pyruvate metabolism; urinary tract infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

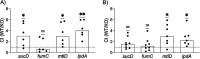

Similar articles
-
Copper Resistance Promotes Fitness of Methicillin-Resistant Staphylococcus aureus during Urinary Tract Infection.mBio. 2021 Oct 26;12(5):e0203821. doi: 10.1128/mBio.02038-21. Epub 2021 Sep 7. mBio. 2021. PMID: 34488457 Free PMC article.
-
Human Urine Alters Methicillin-Resistant Staphylococcus aureus Virulence and Transcriptome.Appl Environ Microbiol. 2021 Jul 27;87(16):e0074421. doi: 10.1128/AEM.00744-21. Epub 2021 Jul 27. Appl Environ Microbiol. 2021. PMID: 34105987 Free PMC article.
-
Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract.Proc Natl Acad Sci U S A. 2017 Oct 10;114(41):E8721-E8730. doi: 10.1073/pnas.1707572114. Epub 2017 Sep 25. Proc Natl Acad Sci U S A. 2017. PMID: 28973850 Free PMC article.
-
Catheter-Associated Urinary Tract Infection, Clostridioides difficile Colitis, Central Line-Associated Bloodstream Infection, and Methicillin-Resistant Staphylococcus aureus.Med Clin North Am. 2020 Jul;104(4):663-679. doi: 10.1016/j.mcna.2020.02.004. Epub 2020 May 12. Med Clin North Am. 2020. PMID: 32505259 Review.
-
Methicillin-resistant Staphylococcus aureus: a controversial food-borne pathogen.Lett Appl Microbiol. 2017 Jun;64(6):409-418. doi: 10.1111/lam.12735. Epub 2017 May 3. Lett Appl Microbiol. 2017. PMID: 28304109 Review.
Cited by
-
Pyruvate Abundance Confounds Aminoglycoside Killing of Multidrug-Resistant Bacteria via Glutathione Metabolism.Research (Wash D C). 2024 Dec 18;7:0554. doi: 10.34133/research.0554. eCollection 2024. Research (Wash D C). 2024. PMID: 39697188 Free PMC article.
-
The NLRP3 Inflammasome Is Dispensable in Methicillin-Resistant Staphylococcus aureus Urinary Tract Infection.Pathogens. 2024 Jan 25;13(2):106. doi: 10.3390/pathogens13020106. Pathogens. 2024. PMID: 38392844 Free PMC article.
-
Rhein against Staphylococcus xylosus by interfering with respiratory metabolism and inducing oxidative stress.Curr Res Food Sci. 2024 Mar 16;8:100718. doi: 10.1016/j.crfs.2024.100718. eCollection 2024. Curr Res Food Sci. 2024. PMID: 38545378 Free PMC article.
References
-
- Shrestha LB, Baral R, Khanal B. 2019. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infect Drug Resist 12:957–963. doi:10.2147/IDR.S200988. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

