Dual Inhibition of KRASG12D and Pan-ERBB Is Synergistic in Pancreatic Ductal Adenocarcinoma
- PMID: 37378556
- PMCID: PMC10502451
- DOI: 10.1158/0008-5472.CAN-23-1313
Dual Inhibition of KRASG12D and Pan-ERBB Is Synergistic in Pancreatic Ductal Adenocarcinoma
Abstract
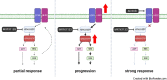
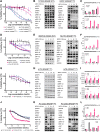
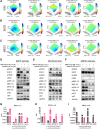
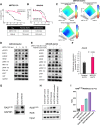
Pancreatic ductal adenocarcinoma (PDAC) is a lethal cancer with a low survival rate. Recently, new drugs that target KRASG12D, a common mutation in PDAC, have been developed. We studied one of these compounds, MRTX1133, and found it was specific and effective at low nanomolar concentrations in patient-derived organoid models and cell lines harboring KRASG12D mutations. Treatment with MRTX1133 upregulated the expression and phosphorylation of EGFR and HER2, indicating that inhibition of ERBB signaling may potentiate MRTX1133 antitumor activity. Indeed, the irreversible pan-ERBB inhibitor, afatinib, potently synergized with MRTX1133 in vitro, and cancer cells with acquired resistance to MRTX1133 in vitro remained sensitive to this combination therapy. Finally, the combination of MRTX1133 and afatinib led to tumor regression and longer survival in orthotopic PDAC mouse models. These results suggest that dual inhibition of ERBB and KRAS signaling may be synergistic and circumvent the rapid development of acquired resistance in patients with KRAS mutant pancreatic cancer.
Significance: KRAS-mutant pancreatic cancer models, including KRAS inhibitor-resistant models, show exquisite sensitivity to combined pan-ERBB and KRAS targeting, which provides the rationale for testing this drug combination in clinical trials.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

