Safety and Tolerability of Intravenous Immunoglobulin in Chronic Inflammatory Demyelinating Polyneuropathy: Results of the ProCID Study
- PMID: 37378806
- PMCID: PMC10442284
- DOI: 10.1007/s40264-023-01326-z
Safety and Tolerability of Intravenous Immunoglobulin in Chronic Inflammatory Demyelinating Polyneuropathy: Results of the ProCID Study
Abstract
Background and aims: The ProCID study evaluated the efficacy and safety of three doses of a 10% liquid intravenous immunoglobulin (IVIg) preparation (panzyga®) in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). This report describes the safety findings.
Methods: Patients were randomised to receive a 2.0 g/kg induction dose followed by maintenance doses of either 0.5, 1.0 or 2.0 g/kg IVIg every 3 weeks over 24 weeks.
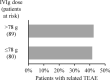
Results: All 142 enrolled patients were included in the safety analyses. In total, 286 treatment-emergent adverse events (TEAEs) were reported in 89 patients, of which 173 (60.5%) were considered treatment-related. Most TEAEs were of mild severity. Eleven serious TEAEs were reported in 6 patients. Two serious TEAEs in one patient (headache and vomiting) were considered related to treatment, which resolved without study discontinuation. No treatment-related thrombotic events, haemolytic transfusion reactions or deaths occurred. One patient discontinued the study due to a TEAE (allergic dermatitis) probably related to IVIg. Headache was the only dose-dependent TEAE, with incidences ranging from 2.9 to 23.7%, the incidence of all other TEAEs was similar across treatment groups. Most TEAEs were associated with the induction dose infusion, and the rate of TEAEs decreased thereafter. The median (IQR) daily IVIg dose was 78 (64-90) g, and 94.4% of patients tolerated the maximal infusion rate of 0.12 ml/kg/min without pre-medication.
Interpretation: Infusions of 10% IVIg at doses up to 2.0 g/kg with high infusion rates were safe and well tolerated in patients with CIDP.
Clinical trial numbers: EudraCT 2015-005443-14, NCT02638207.
© 2023. The Author(s).
Conflict of interest statement
D.R.C. reports consulting for Amgen Inc., Annexon Biosciences, Boehringer Ingelheim, Grifols S.A., Johnson & Johnson, Neura Bio, Novartis, Octapharma AG, Pfizer Inc., Roche, Seattle Genetics Inc., Valenzabio. D.R.C. is on the Data Safety Monitoring Board for the following: PledPharma, Hansa Medical, and Mitsubishi Tanabe Pharma Corporation. D.R.C. is on the Scientific Advisory Board for Sinomab, Algotherapeutics, Nervosave. D.R.C. received royalties for technology licensing from Worldwide Clinical Trials, Inc., CMIC, MedImmune Ltd., RWS Life Sciences, Levicept, AstraZeneca Pharmaceuticals, LP, Genentech, Inc., Chiesi Farmaceutici S.p.A., Beijing 3E-Regenacy Pharmaceuticals Co., Passage Bio, and Disarm Therapeutics, outside the submitted work. P.A.v.D. reports grants from Sanquin Blood Supply Prinses Beatrix Spierfonds, and Takeda during the conduct of the study; and consulting fees from Annexion, Argenx, Octapharma, and Hansa, all outside the submitted work. All grants and consulting fees are transferred to the Erasmus MC Research fund. H.P.H. reports consulting for CSL Behring, Sanofi Genzyme, and UCB. H.P.H. received payments or honoraria from CSL Behring and Octapharma. I.S.J.M. reports grants from Talecris Talents program, GBS/CIDP Foundation International and FP7 EU program, outside the submitted work. Furthermore, a research foundation at the University of Maastricht received honoraria on behalf of him for participation in steering committees of the Talecris Immune Globulin Intravenous For Chronic Inflammatory Demyelinating Polyneuropathy Study, Commonwealth Serum Laboratories, Behring, Octapharma, LFB, Novartis, Union Chimique Belge, Johnson & Johnson, Argenx, outside the submitted work. And Octapharma during the conduct of the study. H.D.K. is on Steering Committees for Octapharma and Sanofi Genzyme. H.D.K. reports travel support and consulting fees from Octapharma in relation to a study design advisory board. H.D.K. reports consulting for UCB, Terumo, Akcea, Alnylam, CSL Behring, Merz, Pfizer, Roche and Dyne. H.D.K. is on Data Safety Monitoring Boards or Advisory Boards for UCB, Syneos and Octapharma. H.D.K. received a grant from Takeda for investigator-initiated research. D.H. and E.C. are employees of Octapharma PPG, Vienna, Austria.
Figures


References
-
- van den Bergh PYK, van Doorn PA, Hadden RDM, Avau B, Vankrunkelsven P, Allen JA, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint Task Force-Second revision. Eur J Neurol. 2021;28:3556–3583. doi: 10.1111/ene.14959. - DOI - PubMed
-
- Hughes RAC, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–144. doi: 10.1016/S1474-4422(07)70329-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

