A Sequential Adaptive Intervention Strategy Targeting Remission and Functional Recovery in Young People at Ultrahigh Risk of Psychosis: The Staged Treatment in Early Psychosis (STEP) Sequential Multiple Assignment Randomized Trial
- PMID: 37378974
- PMCID: PMC10308298
- DOI: 10.1001/jamapsychiatry.2023.1947
A Sequential Adaptive Intervention Strategy Targeting Remission and Functional Recovery in Young People at Ultrahigh Risk of Psychosis: The Staged Treatment in Early Psychosis (STEP) Sequential Multiple Assignment Randomized Trial
Abstract
Importance: Clinical trials have not established the optimal type, sequence, and duration of interventions for people at ultrahigh risk of psychosis.
Objective: To determine the effectiveness of a sequential and adaptive intervention strategy for individuals at ultrahigh risk of psychosis.
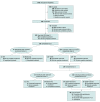
Design, setting, and participants: The Staged Treatment in Early Psychosis (STEP) sequential multiple assignment randomized trial took place within the clinical program at Orygen, Melbourne, Australia. Individuals aged 12 to 25 years who were seeking treatment and met criteria for ultrahigh risk of psychosis according to the Comprehensive Assessment of At-Risk Mental States were recruited between April 2016 and January 2019. Of 1343 individuals considered, 342 were recruited.
Interventions: Step 1: 6 weeks of support and problem solving (SPS); step 2: 20 weeks of cognitive-behavioral case management (CBCM) vs SPS; and step 3: 26 weeks of CBCM with fluoxetine vs CBCM with placebo with an embedded fast-fail option of ω-3 fatty acids or low-dose antipsychotic medication. Individuals who did not remit progressed through these steps; those who remitted received SPS or monitoring for up to 12 months.
Main outcomes and measures: Global Functioning: Social and Role scales (primary outcome), Brief Psychiatric Rating Scale, Scale for the Assessment of Negative Symptoms, Montgomery-Åsberg Depression Rating Scale, quality of life, transition to psychosis, and remission and relapse rates.
Results: The sample comprised 342 participants (198 female; mean [SD] age, 17.7 [3.1] years). Remission rates, reflecting sustained symptomatic and functional improvement, were 8.5%, 10.3%, and 11.4% at steps 1, 2, and 3, respectively. A total of 27.2% met remission criteria at any step. Relapse rates among those who remitted did not significantly differ between SPS and monitoring (step 1: 65.1% vs 58.3%; step 2: 37.7% vs 47.5%). There was no significant difference in functioning, symptoms, and transition rates between SPS and CBCM and between CBCM with fluoxetine and CBCM with placebo. Twelve-month transition rates to psychosis were 13.5% (entire sample), 3.3% (those who ever remitted), and 17.4% (those with no remission).
Conclusions and relevance: In this sequential multiple assignment randomized trial, transition rates to psychosis were moderate, and remission rates were lower than expected, partly reflecting the ambitious criteria set and challenges with real-world treatment fidelity and adherence. While all groups showed mild to moderate functional and symptomatic improvement, this was typically short of remission. While further adaptive trials that address these challenges are needed, findings confirm substantial and sustained morbidity and reveal relatively poor responsiveness to existing treatments.
Trial registration: ClinicalTrials.gov Identifier: NCT02751632.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

