Targeting CCR7-PI3Kγ overcomes resistance to tyrosine kinase inhibitors in ALK-rearranged lymphoma
- PMID: 37379367
- PMCID: PMC10804420
- DOI: 10.1126/scitranslmed.abo3826
Targeting CCR7-PI3Kγ overcomes resistance to tyrosine kinase inhibitors in ALK-rearranged lymphoma
Abstract
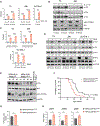
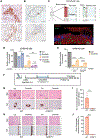
Anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) show potent efficacy in several ALK-driven tumors, but the development of resistance limits their long-term clinical impact. Although resistance mechanisms have been studied extensively in ALK-driven non-small cell lung cancer, they are poorly understood in ALK-driven anaplastic large cell lymphoma (ALCL). Here, we identify a survival pathway supported by the tumor microenvironment that activates phosphatidylinositol 3-kinase γ (PI3K-γ) signaling through the C-C motif chemokine receptor 7 (CCR7). We found increased PI3K signaling in patients and ALCL cell lines resistant to ALK TKIs. PI3Kγ expression was predictive of a lack of response to ALK TKI in patients with ALCL. Expression of CCR7, PI3Kγ, and PI3Kδ were up-regulated during ALK or STAT3 inhibition or degradation and a constitutively active PI3Kγ isoform cooperated with oncogenic ALK to accelerate lymphomagenesis in mice. In a three-dimensional microfluidic chip, endothelial cells that produce the CCR7 ligands CCL19/CCL21 protected ALCL cells from apoptosis induced by crizotinib. The PI3Kγ/δ inhibitor duvelisib potentiated crizotinib activity against ALCL lines and patient-derived xenografts. Furthermore, genetic deletion of CCR7 blocked the central nervous system dissemination and perivascular growth of ALCL in mice treated with crizotinib. Thus, blockade of PI3Kγ or CCR7 signaling together with ALK TKI treatment reduces primary resistance and the survival of persister lymphoma cells in ALCL.
Conflict of interest statement
Figures





References
-
- Gambacorti-Passerini C, Orlov S, Zhang L, Braiteh F, Huang H, Esaki T, Horibe K, Ahn J-S, Beck JT, Edenfield WJ, Shi Y, Taylor M, Tamura K, Van Tine BA, Wu S-J, Paolini J, Selaru P, Kim TM, Long-term effects of crizotinib in ALK-positive tumors (excluding NSCLC): A phase 1b open-label study. Am. J. Hematol 93, 607–614 (2018). - PMC - PubMed
-
- Gambacorti Passerini C, Farina F, Stasia A, Redaelli S, Ceccon M, Mologni L, Messa C, Guerra L, Giudici G, Sala E, Mussolin L, Deeren D, King MH, Steurer M, Ordemann R, Cohen AM, Grube M, Bernard L, Chiriano G, Antolini L, Piazza R, Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients. J. Natl. Cancer Inst 106, djt378 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

