Successful defibrillation testing in patients undergoing elective subcutaneous implantable cardioverter-defibrillator generator replacement
- PMID: 37379530
- PMCID: PMC10325005
- DOI: 10.1093/europace/euad184
Successful defibrillation testing in patients undergoing elective subcutaneous implantable cardioverter-defibrillator generator replacement
Abstract
Aims: After implantation of a subcutaneous implantable cardioverter-defibrillator (S-ICD), a defibrillation test (DFT) is performed to ensure that the device can effectively detect and terminate the induced ventricular arrhythmia. Data on DFT efficacy at generator replacement are scarce with a limited number of patients and conflicting results. This study evaluates conversion efficacy during DFT at elective S-ICD generator replacement in a large cohort from our tertiary centre.
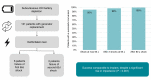
Methods and results: Retrospective data of patients who underwent an S-ICD generator replacement for battery depletion with subsequent DFT between February 2015 and June 2022 were collected. Defibrillation test data were collected from both implant and replacement procedures. PRAETORIAN scores at implant were calculated. Defibrillation test was defined unsuccessful when two conversions at 65 J failed. A total of 121 patients were included. The defibrillation test was successful in 95% after the first and 98% after two consecutive tests. This was comparable with success rates at implant, despite a significant rise in shock impedance (73 ± 23 vs. 83 ± 24 Ω, P < 0.001). Both patients with an unsuccessful DFT at 65 J successfully converted with 80 J.
Conclusion: This study shows a high DFT conversion rate at elective S-ICD generator replacement, which is comparable to conversion rates at implant, despite a rise in shock impedance. Evaluating device position before generator replacement may be recommended to optimize defibrillation success at generator replacement.
Keywords: Defibrillation test; Generator replacement; Impedance; PRAETORIAN score; Subcutaneous ICD.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.E.K. reports consultancy fees and research grants from Abbott, Boston Scientific, Cardiac, and Medtronic and has stock options from AtaCor Medical Inc. A.A.M.W. is a member of the Scientific advisory board of Thryv Therapeutics and ARMGO. K.M.K. reports consultancy fees from Boston Scientific. The other authors report no conflicts of interest.
Figures
Comment on
-
A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable cardioverter-defibrillator: The PRAETORIAN score.Heart Rhythm. 2019 Mar;16(3):403-410. doi: 10.1016/j.hrthm.2018.09.029. Epub 2018 Oct 4. Heart Rhythm. 2019. PMID: 30292861
References
-
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau Ret al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. - PubMed
-
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein Het al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med 1996;335:1933–40. - PubMed
-
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, Boersma LVA, Kuschyk J, El-Chami MFet al. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical