Reflections from the OARSI 2022 clinical trials symposium: The pain of OA-Deconstruction of pain and patient-reported outcome measures for the benefit of patients and clinical trial design
- PMID: 37380011
- PMCID: PMC11184959
- DOI: 10.1016/j.joca.2023.06.006
Reflections from the OARSI 2022 clinical trials symposium: The pain of OA-Deconstruction of pain and patient-reported outcome measures for the benefit of patients and clinical trial design
Abstract
Objective: Osteoarthritis (OA) drug development is hampered by a number of challenges. One of the main challenges is the apparent discordance between pain and structure, which has had a significant impact on drug development programs and has led to hesitance among stakeholders. Since 2017, the Clinical Trials Symposium (CTS) has been hosted under the Osteoarthritis Research Society International (OARSI) leadership. OARSI and the CTS steering committee yearly invite and encourage discussions on selected special subject matter between regulators, drug developers, clinicians, clinical researchers, biomarker specialists, and basic scientists to progress drug development in the OA field.
Method: The main topic for the 2022 OARSI CTS was to elucidate the many facets of pain in OA and to enable a discussion between regulators (Food and Drug Administration (FDA) and the European Medicines Agency (EMA)) and drug developers to clarify outcomes and study designs for OA drug development.
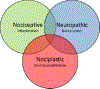
Results: Signs or symptoms indicative of nociceptive pain occur in 50-70% of OA patients, neuropathic-like pain in 15-30% of patients, and nociplastic pain in 15-50% of patients. Weight-bearing knee pain is associated with bone marrow lesions and effusions. There are currently no simple objective functional tests whose improvements correlate with patient perceptions.
Conclusions: The CTS participants, in collaboration with the FDA and EMA, raised several suggestions that they consider key to future clinical trials in OA including the need for more precise differentiation of pain symptoms and mechanisms, and methods to reduce placebo responses in OA trials.
Keywords: Biomarkers; Clinical trial design; EMA; FDA.
Copyright © 2023 Osteoarthritis Research Society International. All rights reserved.
Conflict of interest statement
Conflict of interest None.
Figures
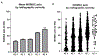

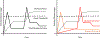

References
-
- Karsdal MA, Tambiah J, Hochberg MC, Ladel C, Bay-Jensen AC, Arendt-Nielsen L, et al. Reflections from the 2021 OARSI clinical trial symposium: Considerations for understanding biomarker assessments in osteoarthritis drug development - Should future studies focus on disease activity, rather than status? Osteoarthr Cartil Open [Internet]. 2022. Sep;4(3):100262. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2665913122000309 - PMC - PubMed
-
- Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord [Internet]. 2008. Sep 2;9:116. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18764949 - PMC - PubMed
-
- Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol [Internet]. 2000. Jun;27(6):1513–7.Available from: http://www.ncbi.nlm.nih.gov/pubmed/10852280 - PubMed
-
- Barr AJ, Campbell TM, Hopkinson D, Kingsbury SR, Bowes MA, Conaghan PG. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res Ther [Internet]. 2015. Aug 25;17:228. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26303219 - PMC - PubMed
-
- Hill CL, Gale DR, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Periarticular lesions detected on magnetic resonance imaging: prevalence in knees with and without symptoms. Arthritis Rheum [Internet]. 2003. Oct;48(10):2836–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14558089 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

