The gut-brain axis mediates bacterial driven modulation of reward signaling
- PMID: 37380023
- PMCID: PMC10372379
- DOI: 10.1016/j.molmet.2023.101764
The gut-brain axis mediates bacterial driven modulation of reward signaling
Abstract
Objective: Our goal is to investigate if microbiota composition modulates reward signaling and assess the role of the vagus in mediating microbiota to brain communication.
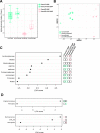
Methods: Male germ-free Fisher rats were colonized with gastrointestinal contents from chow (low fat (LF) ConvLF) or HF (ConvHF) fed rats.
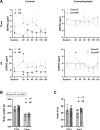
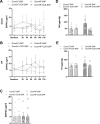
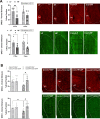
Results: Following colonization, ConvHF rats consumed significantly more food than ConvLF animals. ConvHF rats displayed lower feeding-induced extracellular DOPAC levels (a metabolite of dopamine) in the Nucleus Accumbens (NAc) as well as reduced motivation for HF foods compared to ConvLF rats. Dopamine receptor 2 (DDR2) expression levels in the NAc were also significantly lower in ConvHF animals. Similar deficits were observed in conventionally raised HF fed rats, showing that diet-driven alteration in reward can be initiated via microbiota. Selective gut to brain deafferentation restored DOPAC levels, DRD2 expression, and motivational drive in ConvHF rats.
Conclusions: We concluded from these data that a HF-type microbiota is sufficient to alter appetitive feeding behavior and that bacteria to reward communication is mediated by the vagus nerve.
Keywords: Appetitive behavior; Dopamine; Germ-free rats; Microbiota; Vagus afferent neuron.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures










Similar articles
-
Gut microbiota composition modulates inflammation and structure of the vagal afferent pathway.Physiol Behav. 2020 Oct 15;225:113082. doi: 10.1016/j.physbeh.2020.113082. Epub 2020 Jul 16. Physiol Behav. 2020. PMID: 32682966 Free PMC article.
-
Transfer of microbiota from lean donors in combination with prebiotics prevents excessive weight gain and improves gut-brain vagal signaling in obese rats.Gut Microbes. 2024 Jan-Dec;16(1):2421581. doi: 10.1080/19490976.2024.2421581. Epub 2024 Nov 1. Gut Microbes. 2024. PMID: 39485288 Free PMC article.
-
Manipulation of feeding patterns in high fat diet fed rats improves microbiota composition dynamics, inflammation and gut-brain signaling.Physiol Behav. 2024 Oct 15;285:114643. doi: 10.1016/j.physbeh.2024.114643. Epub 2024 Jul 25. Physiol Behav. 2024. PMID: 39059597
-
Microbiota modulation by eating patterns and diet composition: impact on food intake.Am J Physiol Regul Integr Comp Physiol. 2018 Dec 1;315(6):R1254-R1260. doi: 10.1152/ajpregu.00037.2018. Epub 2018 Sep 19. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30230934 Review.
-
Gut microbes participate in food preference alterations during obesity.Gut Microbes. 2021 Jan-Dec;13(1):1959242. doi: 10.1080/19490976.2021.1959242. Gut Microbes. 2021. PMID: 34424831 Free PMC article. Review.
Cited by
-
Polyunsaturated fatty acids promote appetite via the microbiome-gut-brain axis.bioRxiv [Preprint]. 2025 Jul 29:2025.07.29.667447. doi: 10.1101/2025.07.29.667447. bioRxiv. 2025. PMID: 40766454 Free PMC article. Preprint.
-
Weight Cycling Deregulates Eating Behavior in Mice via the Induction of Durable Gut Dysbiosis.Adv Sci (Weinh). 2025 Aug;12(32):e01214. doi: 10.1002/advs.202501214. Epub 2025 Jun 26. Adv Sci (Weinh). 2025. PMID: 40574486 Free PMC article.
-
TLR4-dependent neuroinflammation mediates LPS-driven food-reward alterations during high-fat exposure.J Neuroinflammation. 2024 Nov 23;21(1):305. doi: 10.1186/s12974-024-03297-z. J Neuroinflammation. 2024. PMID: 39580436 Free PMC article.
-
Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats.Nutrients. 2025 Mar 26;17(7):1147. doi: 10.3390/nu17071147. Nutrients. 2025. PMID: 40218905 Free PMC article.
-
Transfer with microbiota from lean donors prevents excessive weight gain and restores gut-brain vagal signaling in obese rats maintained on a high fat diet.Res Sq [Preprint]. 2024 May 31:rs.3.rs-4438240. doi: 10.21203/rs.3.rs-4438240/v1. Res Sq. 2024. Update in: Gut Microbes. 2024 Jan-Dec;16(1):2421581. doi: 10.1080/19490976.2024.2421581. PMID: 38853960 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

