Bacillus subtilis extracellular protease production incurs a context-dependent cost
- PMID: 37380434
- PMCID: PMC10952608
- DOI: 10.1111/mmi.15110
Bacillus subtilis extracellular protease production incurs a context-dependent cost
Abstract
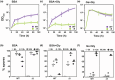
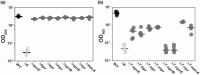
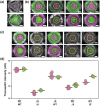
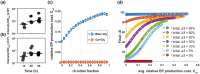
Microbes encounter a wide range of polymeric nutrient sources in various environmental settings, which require processing to facilitate growth. Bacillus subtilis, a bacterium found in the rhizosphere and broader soil environment, is highly adaptable and resilient due to its ability to utilise diverse sources of carbon and nitrogen. Here, we explore the role of extracellular proteases in supporting growth and assess the cost associated with their production. We provide evidence of the essentiality of extracellular proteases when B. subtilis is provided with an abundant, but polymeric nutrient source and demonstrate the extracellular proteases as a shared public good that can operate over a distance. We show that B. subtilis is subjected to a public good dilemma, specifically in the context of growth sustained by the digestion of a polymeric food source. Furthermore, using mathematical simulations, we uncover that this selectively enforced dilemma is driven by the relative cost of producing the public good. Collectively, our findings reveal how bacteria can survive in environments that vary in terms of immediate nutrient accessibility and the consequent impact on the population composition. These findings enhance our fundamental understanding of how bacteria respond to diverse environments, which has importance to contexts ranging from survival in the soil to infection and pathogenesis scenarios.
Keywords: extracellular proteases; nutrient accessibility; public good dilemma.
© 2023 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Arkhipova, T.N. , Veselov, S.U. , Melentiev, A.I. , Martynenko, E.V. & Kudoyarova, G.R. (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant and Soil, 272, 201–209.
-
- Blake, C. , Christensen, M.N. & Kovacs, A.T. (2021) Molecular aspects of plant growth promotion and protection by Bacillus subtilis . Molecular Plant‐Microbe Interactions, 34, 15–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

