Increased glucose availability sensitizes pancreatic cancer to chemotherapy
- PMID: 37380658
- PMCID: PMC10307839
- DOI: 10.1038/s41467-023-38921-8
Increased glucose availability sensitizes pancreatic cancer to chemotherapy
Abstract
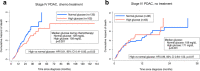
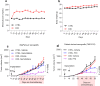
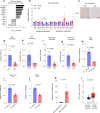
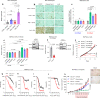
Pancreatic Ductal Adenocarcinoma (PDAC) is highly resistant to chemotherapy. Effective alternative therapies have yet to emerge, as chemotherapy remains the best available systemic treatment. However, the discovery of safe and available adjuncts to enhance chemotherapeutic efficacy can still improve survival outcomes. We show that a hyperglycemic state substantially enhances the efficacy of conventional single- and multi-agent chemotherapy regimens against PDAC. Molecular analyses of tumors exposed to high glucose levels reveal that the expression of GCLC (glutamate-cysteine ligase catalytic subunit), a key component of glutathione biosynthesis, is diminished, which in turn augments oxidative anti-tumor damage by chemotherapy. Inhibition of GCLC phenocopies the suppressive effect of forced hyperglycemia in mouse models of PDAC, while rescuing this pathway mitigates anti-tumor effects observed with chemotherapy and high glucose.
© 2023. The Author(s).
Conflict of interest statement
J.M.S. is a co-author on patents of IDH1 inhibitors, and has received sponsored research funding from the Barer Institute and patents pending to Wistar Institute. C.A.L. has received consulting fees from Astellas Pharmaceuticals and Odyssey Therapeutics, and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in cancer, and targeting the GOT1-pathway as a therapeutic approach. J.M.W. along with University Hospitals filed the following patent application on September 24, 2020: Methods for Treating Wild Type Isocitrate Dehydrogenase 1 Cancers. Information regarding this patent application is as follows: PCT/US20/52445 filed 09/24/20, Claiming Priority to US 62/911,717 filed 10/7/19, File Nos: UHOSP-19738 | 2019-014.
Figures


References
-
- Kou et al. (Cancer Epidemiology Biomarkers & Prevention, 2020).
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA046592/CA/NCI NIH HHS/United States
- R01 CA212600/CA/NCI NIH HHS/United States
- R21 CA173605/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- P50 CA150964/CA/NCI NIH HHS/United States
- F32 CA247466/CA/NCI NIH HHS/United States
- P30 CA043703/CA/NCI NIH HHS/United States
- R00 CA225756/CA/NCI NIH HHS/United States
- R33 DK070291/DK/NIDDK NIH HHS/United States
- R01 CA196643/CA/NCI NIH HHS/United States
- R37 CA237421/CA/NCI NIH HHS/United States
- S10 OD030245/OD/NIH HHS/United States
- U01 CA224012/CA/NCI NIH HHS/United States
- R01 CA248160/CA/NCI NIH HHS/United States
- R21 CA263996/CA/NCI NIH HHS/United States
- R37 CA227865/CA/NCI NIH HHS/United States
- R01 CA244931/CA/NCI NIH HHS/United States
- P30 CA069533/CA/NCI NIH HHS/United States
- R01 CA287672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

