GDF15 promotes weight loss by enhancing energy expenditure in muscle
- PMID: 37380764
- PMCID: PMC10322716
- DOI: 10.1038/s41586-023-06249-4
GDF15 promotes weight loss by enhancing energy expenditure in muscle
Abstract
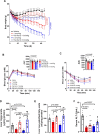
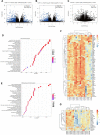
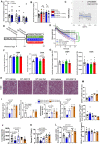
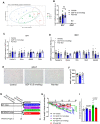
Caloric restriction that promotes weight loss is an effective strategy for treating non-alcoholic fatty liver disease and improving insulin sensitivity in people with type 2 diabetes1. Despite its effectiveness, in most individuals, weight loss is usually not maintained partly due to physiological adaptations that suppress energy expenditure, a process known as adaptive thermogenesis, the mechanistic underpinnings of which are unclear2,3. Treatment of rodents fed a high-fat diet with recombinant growth differentiating factor 15 (GDF15) reduces obesity and improves glycaemic control through glial-cell-derived neurotrophic factor family receptor α-like (GFRAL)-dependent suppression of food intake4-7. Here we find that, in addition to suppressing appetite, GDF15 counteracts compensatory reductions in energy expenditure, eliciting greater weight loss and reductions in non-alcoholic fatty liver disease (NAFLD) compared to caloric restriction alone. This effect of GDF15 to maintain energy expenditure during calorie restriction requires a GFRAL-β-adrenergic-dependent signalling axis that increases fatty acid oxidation and calcium futile cycling in the skeletal muscle of mice. These data indicate that therapeutic targeting of the GDF15-GFRAL pathway may be useful for maintaining energy expenditure in skeletal muscle during caloric restriction.
© 2023. The Author(s).
Conflict of interest statement
S.B.J. and R.E.K. are employees of Novo Nordisk, a pharmaceutical company producing and selling medicine for the treatment of diabetes and obesity. G.R.S. is a co-founder and shareholder of Espervita Therapeutics. McMaster University has received funding from Espervita Therapeutics, Esperion Therapeutics, Poxel Pharmaceuticals and Nestle for research conducted in the laboratory of G.R.S. S.R. is supported by a MITACS postdoctoral fellowship sponsored by Novo Nordisk. H.C.G. holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. G.R.S., G.P. and H.C.G. are inventors listed on a patent for identifying GDF15 as a biomarker for metformin. G.R.S. has received consulting/speaking fees from Astra Zeneca, Eli Lilly, Esperion Therapeutics, Merck, Poxel Pharmaceuticals and Cambrian Biosciences. The other authors declare no competing interests.
Figures











Comment in
-
GDF15 boosts muscle energy burn.Nat Rev Endocrinol. 2023 Sep;19(9):499. doi: 10.1038/s41574-023-00877-6. Nat Rev Endocrinol. 2023. PMID: 37452212 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

