Wake-like skin patterning and neural activity during octopus sleep
- PMID: 37380770
- PMCID: PMC10322707
- DOI: 10.1038/s41586-023-06203-4
Wake-like skin patterning and neural activity during octopus sleep
Abstract
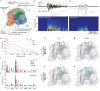
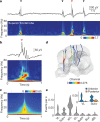
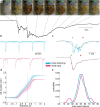
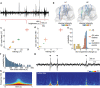
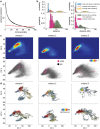
While sleeping, many vertebrate groups alternate between at least two sleep stages: rapid eye movement and slow wave sleep1-4, in part characterized by wake-like and synchronous brain activity, respectively. Here we delineate neural and behavioural correlates of two stages of sleep in octopuses, marine invertebrates that evolutionarily diverged from vertebrates roughly 550 million years ago (ref. 5) and have independently evolved large brains and behavioural sophistication. 'Quiet' sleep in octopuses is rhythmically interrupted by approximately 60-s bouts of pronounced body movements and rapid changes in skin patterning and texture6. We show that these bouts are homeostatically regulated, rapidly reversible and come with increased arousal threshold, representing a distinct 'active' sleep stage. Computational analysis of active sleep skin patterning reveals diverse dynamics through a set of patterns conserved across octopuses and strongly resembling those seen while awake. High-density electrophysiological recordings from the central brain reveal that the local field potential (LFP) activity during active sleep resembles that of waking. LFP activity differs across brain regions, with the strongest activity during active sleep seen in the superior frontal and vertical lobes, anatomically connected regions associated with learning and memory function7-10. During quiet sleep, these regions are relatively silent but generate LFP oscillations resembling mammalian sleep spindles11,12 in frequency and duration. The range of similarities with vertebrates indicates that aspects of two-stage sleep in octopuses may represent convergent features of complex cognition.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Ookawa T, Gotoh J. Electroencephalographs study of chickens: periodic recurrence of low voltage and fast waves during behavioral sleep. Poult. Sci. 1964;43:1603–1604. doi: 10.3382/ps.0431603. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

