Femtosecond proton transfer in urea solutions probed by X-ray spectroscopy
- PMID: 37380782
- PMCID: PMC10371863
- DOI: 10.1038/s41586-023-06182-6
Femtosecond proton transfer in urea solutions probed by X-ray spectroscopy
Abstract
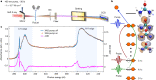
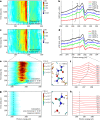
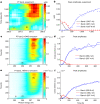
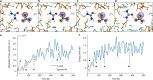
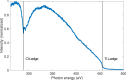
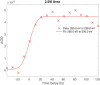
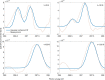
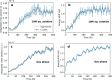
Proton transfer is one of the most fundamental events in aqueous-phase chemistry and an emblematic case of coupled ultrafast electronic and structural dynamics1,2. Disentangling electronic and nuclear dynamics on the femtosecond timescales remains a formidable challenge, especially in the liquid phase, the natural environment of biochemical processes. Here we exploit the unique features of table-top water-window X-ray absorption spectroscopy3-6 to reveal femtosecond proton-transfer dynamics in ionized urea dimers in aqueous solution. Harnessing the element specificity and the site selectivity of X-ray absorption spectroscopy with the aid of ab initio quantum-mechanical and molecular-mechanics calculations, we show how, in addition to the proton transfer, the subsequent rearrangement of the urea dimer and the associated change of the electronic structure can be identified with site selectivity. These results establish the considerable potential of flat-jet, table-top X-ray absorption spectroscopy7,8 in elucidating solution-phase ultrafast dynamics in biomolecular systems.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

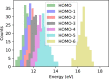

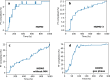
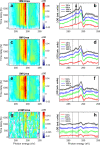

References
-
- Hammes-Schiffer S, Tully JC. Proton transfer in solution: molecular dynamics with quantum transitions. J. Chem. Phys. 1994;101:4657–4667. doi: 10.1063/1.467455. - DOI
-
- Elsässer, T. & Becker, H. Ultrafast Hydrogen Bonding Dynamics and Proton Transfer Processes in the Condensed Phase Vol. 23 (Springer Science & Business Media, 2013).
-
- Saito N, et al. Real-time observation of electronic, vibrational, and rotational dynamics in nitric oxide with attosecond soft x-ray pulses at 400 ev. Optica. 2019;6:1542–1546. doi: 10.1364/OPTICA.6.001542. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

