Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: a blinded controlled multicenter randomized trial
- PMID: 37380908
- PMCID: PMC10562315
- DOI: 10.1007/s00405-023-08085-8
Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: a blinded controlled multicenter randomized trial
Abstract
Purpose: Few evidence-based therapies are available for chronic olfactory dysfunction after COVID-19. This study investigated the relative efficacy of olfactory training alone, co-ultramicronized palmitoylethanolamide with luteolin (um-PEA-LUT, an anti-neuroinflammatory supplement) alone, or combined therapy for treating chronic olfactory dysfunction from COVID-19.
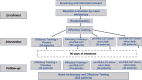
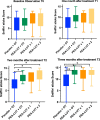
Methods: This double-blinded controlled, placebo-controlled multicenter randomized clinical trial was conducted in 202 patients with persistent COVID-19 olfactory dysfunction of > 6 month duration. After a screening nasal endoscopy, patients were randomized to: (1) olfactory training and placebo; (2) once daily um-PEA-LUT alone; (3) twice daily um-PEA-LUT alone; or (4) combination of once daily um-PEA-LUT with olfactory training. Olfactory testing (Sniffin' Sticks odor identification test) was performed at baseline and at 1, 2, and 3 months. The primary outcome was recovery of over three points on olfactory testing, with outcomes compared at T0, T1, T2 and T3 across groups. Statistical analyses included one-way ANOVA for numeric data and chi-square for nominal data.
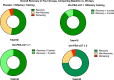
Results: All patients completed the study, and there were no adverse events. At 90 days, odor identification scores improved by > 3 points in 89.2% of patients receiving combined therapy vs. 36.8% receiving olfactory training with placebo, 40% receiving twice daily um-PEA-LUT alone, and 41.6% receiving once daily um-PEA-LUT alone (p < 0.00001). Patients receiving treatment with um-PEA-LUT alone demonstrated subclinical improvement (< 3 point odor identification improvement) more often than patients receiving olfactory training with placebo (p < 0.0001.) CONCLUSIONS: Olfactory training plus once daily um-PEA-LUT resulted in greater olfactory recovery than either therapy alone in patients with long-term olfactory function due to COVID-19.
Trial registration: 20112020PGFN on clinicaltrials.gov.
Level of evidence: 1b (Individual Randomized Clinical Trial).
Keywords: Anosmia; COVID-19; Clinical trial; Coronavirus; Hyposmia; Luteolin; Neuroinflammation; Olfactory training; PEA; PEA–LUT; Palmitoylethanolamide; Post-acute sequelae of COVID-19; Randomized trial; SARS-CoV-2; Smell disorders.
© 2023. The Author(s).
Conflict of interest statement
The authors have no disclosures.
Figures
Similar articles
-
Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial.Curr Neuropharmacol. 2022;20(10):2001-2012. doi: 10.2174/1570159X20666220420113513. Curr Neuropharmacol. 2022. PMID: 35450527 Free PMC article. Clinical Trial.
-
Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)?Life (Basel). 2023 Jan 13;13(1):226. doi: 10.3390/life13010226. Life (Basel). 2023. PMID: 36676175 Free PMC article.
-
Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study.Cells. 2022 Aug 17;11(16):2552. doi: 10.3390/cells11162552. Cells. 2022. PMID: 36010630 Free PMC article. Clinical Trial.
-
Investigational drugs for the treatment of olfactory dysfunction.Expert Opin Investig Drugs. 2022 Sep;31(9):945-955. doi: 10.1080/13543784.2022.2113054. Epub 2022 Aug 19. Expert Opin Investig Drugs. 2022. PMID: 35983993
-
Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies.PLoS One. 2023 Aug 2;18(8):e0288285. doi: 10.1371/journal.pone.0288285. eCollection 2023. PLoS One. 2023. PMID: 37531338 Free PMC article.
Cited by
-
Polyunsaturated Fatty Acids as Potential Treatments for COVID-19-Induced Anosmia.Biomedicines. 2024 Sep 12;12(9):2085. doi: 10.3390/biomedicines12092085. Biomedicines. 2024. PMID: 39335598 Free PMC article. Review.
-
Inflammatory pathways in patients with post-acute sequelae of COVID-19: The role of the clinical immunologist.Ann Allergy Asthma Immunol. 2024 Nov;133(5):507-515. doi: 10.1016/j.anai.2024.08.021. Epub 2024 Aug 22. Ann Allergy Asthma Immunol. 2024. PMID: 39179099 Review.
-
Palmitoylethanolamide, an endogenous fatty acid amide, and its pleiotropic health benefits: A narrative review.J Biomed Res. 2024 Oct 22;39(3):215-228. doi: 10.7555/JBR.38.20240053. J Biomed Res. 2024. PMID: 39433509 Free PMC article.
-
Protocol for olfactory training in persisting COVID-19-associated loss of smell (SMELL): a monocentric randomised controlled trial conducted in Innsbruck.BMJ Open. 2025 May 27;15(5):e094027. doi: 10.1136/bmjopen-2024-094027. BMJ Open. 2025. PMID: 40436445 Free PMC article.
-
Investigating the efficacy of melatonin, topical sodium citrate, and multivitamin with zinc as a potential treatment for postinfectious loss of smell.Braz J Otorhinolaryngol. 2024 Nov-Dec;90(6):101496. doi: 10.1016/j.bjorl.2024.101496. Epub 2024 Aug 31. Braz J Otorhinolaryngol. 2024. PMID: 39243697 Free PMC article.
References
-
- Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, Russo E, Costantino A, Pirola F, Cugini G, Malvezzi L, Morenghi E, Azzolini E, Lagioia M, Spriano G. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8):723–728. doi: 10.1001/jamaoto.2020.1155. - DOI - PMC - PubMed
-
- Ferreli F, Gaino F, Russo E, Di Bari M, Rossi V, De Virgilio A, Malvezzi L, Colombo G, Cristalli G, Spriano G, Mercante G. Long-standing gustatory and olfactory dysfunction in COVID-19 patients: a prospective study. Eur Arch Otorhinolaryngol. 2022;279(9):4633–4640. doi: 10.1007/s00405-022-07428-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

