Intrahepatic macrophage reprogramming associated with lipid metabolism in hepatitis B virus-related acute-on-chronic liver failure
- PMID: 37380987
- PMCID: PMC10303321
- DOI: 10.1186/s12967-023-04294-1
Intrahepatic macrophage reprogramming associated with lipid metabolism in hepatitis B virus-related acute-on-chronic liver failure
Abstract
Background: Acute-on-chronic liver failure (ACLF) is a severe syndrome with high short-term mortality, but the pathophysiology still remains largely unknown. Immune dysregulation and metabolic disorders contribute to the progression of ACLF, but the crosstalk between immunity and metabolism during ACLF is less understood. This study aims to depict the immune microenvironment in the liver during ACLF, and explore the role of lipid metabolic disorder on immunity.
Methods: Single-cell RNA-sequencing (scRNA-seq) was performed using the liver non-parenchymal cells (NPCs) and peripheral blood mononuclear cells (PBMCs) from healthy controls, cirrhosis patients and ACLF patients. A series of inflammation-related cytokines and chemokines were detected using liver and plasma samples. The lipid metabolomics targeted free fatty acids (FFAs) in the liver was also detected.
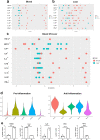
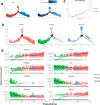
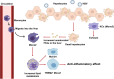
Results: The scRNA-seq analysis of liver NPCs showed a significant increase of monocytes/macrophages (Mono/Mac) infiltration in ACLF livers, whereas the resident Kupffer cells (KCs) were exhausted. A characterized TREM2+ Mono/Mac subpopulation was identified in ACLF, and showed immunosuppressive function. Combined with the scRNA-seq data from PBMCs, the pseudotime analysis revealed that the TREM2+ Mono/Mac were differentiated from the peripheral monocytes and correlated with lipid metabolism-related genes including APOE, APOC1, FABP5 and TREM2. The targeted lipid metabolomics proved the accumulation of unsaturated FFAs associated with α-linolenic acid (α-LA) and α-LA metabolism and beta oxidation of very long chain fatty acids in the ACLF livers, indicating that unsaturated FFAs might promote the differentiation of TREM2+ Mono/Mac during ACLF.
Conclusions: The reprogramming of macrophages was found in the liver during ACLF. The immunosuppressive TREM2+ macrophages were enriched in the ACLF liver and contributed to the immunosuppressive hepatic microenvironment. The accumulation of unsaturated FFAs in the ACLF liver promoted the reprogramming of the macrophages. It might be a potential target to improve the immune deficiency of ACLF patients through regulating lipid metabolism.
Keywords: Acute-on-chronic liver failure; Cirrhosis-associated immune dysfunction; Free fatty acids; Hepatitis B virus; Lipid metabolomics; TREM2; scRNA-seq.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

