The homodimer interfaces of costimulatory receptors B7 and CD28 control their engagement and pro-inflammatory signaling
- PMID: 37381064
- PMCID: PMC10308680
- DOI: 10.1186/s12929-023-00941-3
The homodimer interfaces of costimulatory receptors B7 and CD28 control their engagement and pro-inflammatory signaling
Abstract
Background: The inflammatory response is indispensable for protective immunity, yet microbial pathogens often trigger an excessive response, 'cytokine storm', harmful to the host. Full T-cell activation requires interaction of costimulatory receptors B7-1(CD80) and B7-2(CD86) expressed on antigen-presenting cells with CD28 expressed on the T cells. We created short peptide mimetics of the homodimer interfaces of the B7 and CD28 receptors and examined their ability to attenuate B7/CD28 coligand engagement and signaling through CD28 for inflammatory cytokine induction in human immune cells, and to protect from lethal toxic shock in vivo.
Methods: Short B7 and CD28 receptor dimer interface mimetic peptides were synthesized and tested for their ability to attenuate the inflammatory cytokine response of human peripheral blood mononuclear cells, as well as for their ability to attenuate B7/CD28 intercellular receptor engagement. Mice were used to test the ability of such peptides to protect from lethal superantigen toxin challenge when administered in molar doses far below the toxin dose.
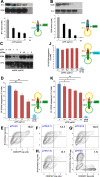
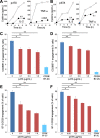
Results: B7 and CD28 homodimer interfaces are remote from the coligand binding sites, yet our finding is that by binding back into the receptor dimer interfaces, short dimer interface mimetic peptides inhibit intercellular B7-2/CD28 as well as the tighter B7-1/CD28 engagement, attenuating thereby pro-inflammatory signaling. B7 mimetic peptides exhibit tight selectivity for the cognate receptor in inhibiting intercellular receptor engagement with CD28, yet each diminishes signaling through CD28. In a prominent example of inflammatory cytokine storm, by attenuating formation of the B7/CD28 costimulatory axis, B7-1 and CD28 dimer interface mimetic peptides protect mice from lethal toxic shock induced by a bacterial superantigen even when administered in doses far submolar to the superantigen.
Conclusions: Our results reveal that the B7 and CD28 homodimer interfaces each control B7/CD28 costimulatory receptor engagement and highlight the protective potential against cytokine storm of attenuating, yet not ablating, pro-inflammatory signaling via these receptor domains.
Keywords: Control of B7/CD28 receptor engagement; Costimulatory receptors B7 and CD28; Inflammatory cytokine storm; Pro-inflammatory signaling; Receptor homodimer interface mimetic peptides; Regulation of B7/CD28 signaling.
© 2023. The Author(s).
Conflict of interest statement
RK, GA and RL are inventors on patents and patent applications for peptides described.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

