Pulsed stimulated Brillouin microscopy
- PMID: 37381389
- PMCID: PMC10316751
- DOI: 10.1364/OE.489158
Pulsed stimulated Brillouin microscopy
Abstract
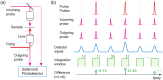
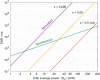
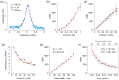
Stimulated Brillouin scattering is an emerging technique for probing the mechanical properties of biological samples. However, the nonlinear process requires high optical intensities to generate sufficient signal-to-noise ratio (SNR). Here, we show that the SNR of stimulated Brillouin scattering can exceed that of spontaneous Brillouin scattering with the same average power levels suitable for biological samples. We verify the theoretical prediction by developing a novel scheme using low duty cycle, nanosecond pulses for the pump and probe. A shot noise-limited SNR over 1000 was measured with a total average power of 10 mW for 2 ms or 50 mW for 200 µs integration on water samples. High-resolution maps of Brillouin frequency shift, linewidth, and gain amplitude from cells in vitro are obtained with a spectral acquisition time of 20 ms. Our results demonstrate the superior SNR of pulsed stimulated Brillouin over spontaneous Brillouin microscopy.
Conflict of interest statement
D.C. and S.H.Y. hold patents on the related technology. The authors declare no competing interests.
Figures



References
-
- Margueritat J., Virgone-Carlotta A., Monnier S., Delanoë-Ayari H., Mertani H. C., Berthelot A., Martinet Q., Dagany X., Rivière C., Rieu J.-P., Dehoux T., “High-Frequency Mechanical Properties of Tumors Measured by Brillouin Light Scattering,” Phys. Rev. Lett. 122(1), 018101 (2019).10.1103/PhysRevLett.122.018101 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

