Biological insights in non-small cell lung cancer
- PMID: 37381723
- PMCID: PMC10466437
- DOI: 10.20892/j.issn.2095-3941.2023.0108
Biological insights in non-small cell lung cancer
Abstract
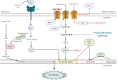
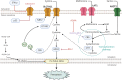
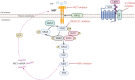
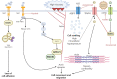
Lung oncogenesis relies on intracellular cysteine to overcome oxidative stress. Several tumor types, including non-small cell lung cancer (NSCLC), upregulate the system xc- cystine/glutamate antiporter (xCT) through overexpression of the cystine transporter SLC7A11, thus sustaining intracellular cysteine levels to support glutathione synthesis. Nuclear factor erythroid 2-related factor 2 (NRF2) serves as a master regulator of oxidative stress resistance by regulating SLC7A11, whereas Kelch-like ECH-associated protein (KEAP1) acts as a cytoplasmic repressor of the oxidative responsive transcription factor NRF2. Mutations in KEAP1/NRF2 and p53 induce SLC7A11 activation in NSCLC. Extracellular cystine is crucial in supplying the intracellular cysteine levels necessary to combat oxidative stress. Disruptions in cystine availability lead to iron-dependent lipid peroxidation, thus resulting in a type of cell death called ferroptosis. Pharmacologic inhibitors of xCT (either SLC7A11 or GPX4) induce ferroptosis of NSCLC cells and other tumor types. When cystine uptake is impaired, the intracellular cysteine pool can be sustained by the transsulfuration pathway, which is catalyzed by cystathionine-B-synthase (CBS) and cystathionine g-lyase (CSE). The involvement of exogenous cysteine/cystine and the transsulfuration pathway in the cysteine pool and downstream metabolites results in compromised CD8+ T cell function and evasion of immunotherapy, diminishing immune response and potentially reducing the effectiveness of immunotherapeutic interventions. Pyroptosis is a previously unrecognized form of regulated cell death. In NSCLCs driven by EGFR, ALK, or KRAS, selective inhibitors induce pyroptotic cell death as well as apoptosis. After targeted therapy, the mitochondrial intrinsic apoptotic pathway is activated, thus leading to the cleavage and activation of caspase-3. Consequently, gasdermin E is activated, thus leading to permeabilization of the cytoplasmic membrane and cell-lytic pyroptosis (indicated by characteristic cell membrane ballooning). Breakthroughs in KRAS G12C allele-specific inhibitors and potential mechanisms of resistance are also discussed herein.
Keywords: KRAS G12C allele-specific inhibitors; Solute carrier family 7 member 11 (SLC7A11); ferroptosis; non-small cell lung cancer (NSCLC); nuclear factor erythroid 2-related factor 2 (NRF2); pyroptosis.
Copyright © 2023 Cancer Biology & Medicine.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures
References
-
- Jaiyesimi IA, Owen DH, Ismaila N, Blanchard E, Celano P, Florez N, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO living guideline, version 2022.3. J Clin Oncol. 2023:Jco2202783 - PubMed
-
- Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–95. - PubMed
-
- Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R, et al. Sugemalimab vs. placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23:220–33. - PubMed
-
- Jaiyesimi IA, Owen DH, Ismaila N, Blanchard E, Celano P, Florez N, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO living guideline, version 2022.3. J Clin Oncol. 2023;41:e31–e41. - PubMed
-
- de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, et al. Sotorasib vs. docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401:733–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
