Self-guided digital acceptance and commitment therapy for fibromyalgia management: results of a randomized, active-controlled, phase II pilot clinical trial
- PMID: 37382794
- PMCID: PMC10867073
- DOI: 10.1007/s10865-023-00429-3
Self-guided digital acceptance and commitment therapy for fibromyalgia management: results of a randomized, active-controlled, phase II pilot clinical trial
Abstract
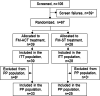
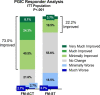
Although empirically validated for fibromyalgia (FM), cognitive and behavioral therapies, including Acceptance and Commitment Therapy (ACT), are inaccessible to many patients. A self-guided, smartphone-based ACT program would significantly improve accessibility. The SMART-FM study assessed the feasibility of conducting a predominantly virtual clinical trial in an FM population in addition to evaluating preliminary evidence for the safety and efficacy of a digital ACT program for FM (FM-ACT). Sixty-seven patients with FM were randomized to 12 weeks of FM-ACT (n = 39) or digital symptom tracking (FM-ST; n = 28). The study population was 98.5% female, with an average age of 53 years and an average baseline FM symptom severity score of 8 out of 11. Endpoints included the Fibromyalgia Impact Questionnaire-Revised (FIQ-R) and the Patient Global Impression of Change (PGIC). The between-arm effect size for the change from baseline to Week 12 in FIQ-R total scores was d = 0.44 (least-squares mean difference, - 5.7; SE, 3.16; 95% CI, - 11.9 to 0.6; P = .074). At Week 12, 73.0% of FM-ACT participants reported improvement on the PGIC versus 22.2% of FM-ST participants (P < .001). FM-ACT demonstrated improved outcomes compared to FM-ST, with high engagement and low attrition in both arms. Retrospectively registered at ClinicalTrials.gov (NCT05005351) on August 13, 2021.
Keywords: Acceptance and Commitment Therapy; Chronic pain; Digital ACT; Fibromyalgia; Prescription digital therapeutic; Smartphone-delivered ACT; Smartphone-delivered CBT.
© 2023. The Author(s).
Conflict of interest statement
S.C. is a paid consultant of Swing Therapeutics, Inc. R.M.G. is a paid consultant of and shareholder in Swing Therapeutics, Inc. A.C.K., N.V., and M.J.R. are employees of and shareholders in Swing Therapeutics, Inc. S.S. and S.M. have no declared conflicts of interest. J.V.L., L.M.M., and D.A.W. are paid advisors of Swing Therapeutics, Inc. L.M.A. is an advisor for and has received research funding from Swing Therapeutics, Inc.
Figures


References
-
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
-
- Bedson J, Hill J, White D, Chen Y, Wathall S, Dent S, Cooke K, van der Windt D. Development and validation of a pain monitoring app for patients with musculoskeletal conditions (the Keele pain recorder feasibility study) BMC Med Inform Decis Mak. 2019;19(1):24. doi: 10.1186/s12911-019-0741-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

