Impact of chronic kidney disease and diabetes on clinical outcomes in women undergoing PCI
- PMID: 37382924
- PMCID: PMC10436070
- DOI: 10.4244/EIJ-D-23-00086
Impact of chronic kidney disease and diabetes on clinical outcomes in women undergoing PCI
Abstract
Background: For women undergoing drug-eluting stent (DES) implantation, the individual and combined impact of chronic kidney disease (CKD) and diabetes mellitus (DM) on outcomes is uncertain.
Aims: We sought to assess the impact of CKD and DM on prognosis in women after DES implantation.
Methods: We pooled patient-level data on women from 26 randomised controlled trials comparing stent types. Women receiving DES were stratified into 4 groups based on CKD (defined as creatine clearance <60 mL/min) and DM status. The primary outcome at 3 years after percutaneous coronary intervention was the composite of all-cause death or myocardial infarction (MI); secondary outcomes included cardiac death, stent thrombosis and target lesion revascularisation.
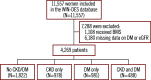
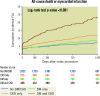
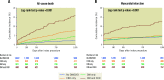
Results: Among 4,269 women, 1,822 (42.7%) had no CKD/DM, 978 (22.9%) had CKD alone, 981 (23.0%) had DM alone, and 488 (11.4%) had both conditions. The risk of all-cause death or MI was not increased in women with CKD alone (adjusted hazard ratio [adj. HR] 1.19, 95% confidence interval [CI]: 0.88-1.61) nor DM alone (adj. HR 1.27, 95% CI: 0.94-1.70), but was significantly higher in women with both conditions (adj. HR 2.64, 95% CI: 1.95-3.56; interaction p-value <0.001). CKD and DM in combination were associated with an increased risk of all secondary outcomes, whereas alone, each condition was only associated with all-cause death and cardiac death.
Conclusions: Among women receiving DES, the combined presence of CKD and DM was associated with a higher risk of the composite of death or MI and of any secondary outcome, whereas alone, each condition was associated with an increase in all-cause and cardiac death.
Conflict of interest statement
A. Spirito received a research grant from the Swiss National Science Foundation (SNSF). R.V. Jeger reports research and educational grants to the institution from Abbott, Amgen, AstraZeneca, Bayer, Biosense Webster, B. Braun Melsungen AG, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cordis, Daiichi Sankyo, Edwards Lifesciences, GE Medical Systems, MCM Medsys, Medtronic, Novartis, Pfizer, Terumo, and Vascular Medical GmbH. G.W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, and Abbott; has served as a consultant to Daiichi Sankyo, Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, and Millennia Biopharma; and has equity/options from Ancora, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter; his daughter is an employee at IQVIA. G.W. Stone’s employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Philips, Biosense-Webster, Shockwave, Vascular Dynamics, Pulnovo, and V-Wave. P.G. Steg reports receiving research grants from Amarin, Bayer, Sanofi, and Servier; compensation for work on clinical trials (steering committee, clinical events committee or data safety and monitoring board) from Amarin, AstraZeneca, Bayer, Bristol-Myers Squibb, Idorsia, Novartis, PhaseBio, Pfizer, Sanofi, and Servier; for consulting or speaking from Amarin, Amgen, BMS/Myokardia, Merck, Novo Nordisk, and Regeneron; and is a Senior Associate Editor at Circulation. S. Windecker reports research, travel or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, CardioValve, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Janssen-Cilag, Johnson & Johnson, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Suisse, Pfizer, Polares, Regeneron, Sanofi-Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; and serves as advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, Janssen, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, V-Wave, and Xeltis, with payments to the institution but no personal payments; he is also member of the steering/executive committee group of several investigator-initiated trials that receive funding from industry without impact on his personal remuneration. W. Wijns received institutional research grants and honoraria from MicroPort (Steering Committee TARGET-AC trial); is co-founder of Argonauts, an innovation facilitator; and is a medical adviser to Rede Optimus Research and Corrib Core Laboratory, NUI Galway. P.W. Serruys reports consultancy payments from Meril Life Sciences, Novartis, Philips, SMT, and Xeltic. M. Valgimigli reports grants and personal fees from Terumo; and personal fees from Abbott Vascular, Alvimedica/CID, AstraZeneca, Bayer, Biotronik, Boston Scientific, Bristol-Myers Squibb, Chiesi, CorFlow, Daiichi Sankyo, Idorsia Pharmaceuticals, Medtronic, Novartis, PhaseBio, Universität Basel (Department of Klinische Forschung), Vesalio, and Vifor. M-C. Morice is CEO and a shareholder of CERC (a CRO based in Massy, France) and also owns shares in Electroducer. B. Pileggi received research grant from the Brazilian Federal Foundation for Support and Evaluation of Graduate Education (CAPES). G. Weisz is a member of the medical advisory board for Filterlex, Intratech, Microbot, and Trisol; received equity from Filterlex, Intratech, and Microbot; and consulting fees from Cuspa, Filterlex, Intratech, Magenta, and Microbot. D.E. Kandzari reports institutional research/grant support from Ablative Solutions and Medtronic; and personal consulting honoraria from Medtronic. A. Kastrati is listed as an inventor in the patent application ─ PCT/EP2021/053116 “Calcium channel TRPC6 inhibitors using balloons, stents or other medical devices” ─ from his institution. C. von Birgelen reports institutional research grants (Thoraxcentrum Twente) from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. G.D. Dangas reports institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Boston Scientific, Medtronic, and Daiichi Sankyo; consultant fees from Biosensors and Boston Scientific; and speaker honoraria from Chiesi. P.C. Smits received institutional grants from Abbott Vascular, SMT and MicroPort; and received consultancy or speakers fees from Abbott Vascular, Abiomed, MicroPort, Terumo, Daiichi Sankyo, and AstraZeneca. T. Kimura reports research grants from Abbott Vascular and Boston Scientific. G.W. Mikhail is Course Director of the Imperial Valve and Cardiovascular Course (IVCC) to which Edwards Lifesciences is a major financial sponsor. A. Chieffo has received speaker fees from Abiomed and GADA. R. Mehran reports institutional research grants from Abbott, Abiomed, Applied Therapeutics, Arena, AstraZeneca, Bayer, Biosensors, Boston Scientific, Bristol-Myers Squibb, CardiaWave, CellAegis, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Insel Gruppe AG, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Philips, Transverse Medical, and Zoll; personal fees from ACC, Boston Scientific, California Institute for Regenerative Medicine (CIRM), Cine-Med Research, Janssen, WebMD, and SCAI; consulting fees paid to the institution from Abbott, Abiomed, AM-Pharma, Alleviant Medical, Bayer, Beth Israel Deaconess, CardiaWave, CeloNova, Chiesi, Concept Medical, DSI, Duke University, Idorsia Pharmaceuticals, Medtronic, Novartis, and Philips; has equity <1% in Applied Therapeutics, Elixir Medical, STEL, and CONTROLRAD (spouse); and serves on the scientific advisory board for AMA and Biosensors (spouse). The other authors have no conflicts of interest to declare.
Figures

References
-
- Kedhi E, Généreux P, Palmerini T, McAndrew TC, Parise H, Mehran R, Dangas GD, Stone GW. Impact of coronary lesion complexity on drug-eluting stent outcomes in patients with and without diabetes mellitus: analysis from 18 pooled randomized trials. J Am Coll Cardiol. 2014;63:2111–8. - PubMed
-
- Lee JM, Kang J, Lee E, Hwang D, Rhee TM, Park J, Kim HL, Lee SE, Han JK, Yang HM, Park KW, Na SH, Kang HJ, Koo BK, Kim HS. Chronic Kidney Disease in the Second-Generation Drug-Eluting Stent Era: Pooled Analysis of the Korean Multicenter Drug-Eluting Stent Registry. JACC Cardiovasc Interv. 2016;9:2097–109. - PubMed
-
- Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet. 2017;389:1238–52. - PubMed
-
- Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14:361–77. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

