Kaliuresis and Intracellular Uptake of Potassium with Potassium Citrate and Potassium Chloride Supplements: A Randomized Controlled Trial
- PMID: 37382933
- PMCID: PMC10578626
- DOI: 10.2215/CJN.0000000000000228
Kaliuresis and Intracellular Uptake of Potassium with Potassium Citrate and Potassium Chloride Supplements: A Randomized Controlled Trial
Abstract
Background: A potassium replete diet is associated with lower cardiovascular risk but may increase the risk of hyperkalemia, particularly in people using renin-angiotensin-aldosterone system inhibitors. We investigated whether intracellular uptake and potassium excretion after an acute oral potassium load depend on the accompanying anion and/or aldosterone and whether this results in altered plasma potassium change.
Methods: In this placebo-controlled interventional cross-over trial including 18 healthy individuals, we studied the acute effects of one oral load of potassium citrate (40 mmol), potassium chloride (40 mmol), and placebo in random order after overnight fasting. Supplements were administered after a 6-week period with and without lisinopril pretreatment. Linear mixed effect models were used to compare blood and urine values before and after supplementation and between the interventions. Univariable linear regression was used to determine the association between baseline variables and change in blood and urine values after supplementation.
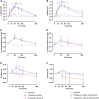
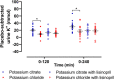
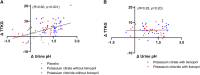
Results: During the 4-hour follow-up, the rise in plasma potassium was similar for all interventions. After potassium citrate, both red blood cell potassium-as measure of the intracellular potassium-and transtubular potassium gradient (TTKG)-reflecting potassium secretory capacity-were higher than after potassium chloride or potassium citrate with lisinopril pretreatment. Baseline aldosterone was significantly associated with TTKG after potassium citrate, but not after potassium chloride or potassium citrate with lisinopril pretreatment. The observed TTKG change after potassium citrate was significantly associated with urine pH change during this intervention ( R =0.60, P < 0.001).
Conclusions: With similar plasma potassium increase, red blood cell potassium uptake and kaliuresis were higher after an acute load of potassium citrate as compared with potassium chloride alone or pretreatment with lisinopril.
Clinical trial registry name and registration number: Potassium supplementation in patients with chronic kidney disease and healthy subjects: effects on potassium and sodium balance, NL7618.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
A.H.J. Danser reports research funding from Alnylam Pharmaceuticals. M.H. De Borst reports consultancy for Astellas, AstraZeneca, Kyowa Kirin, Pharmacosmos, Sanofi Genzyme, and Vifor Pharma; research funding from Sanofi Genzyme and Vifor Pharma; and role as an Associate Editor of
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

