A novel ACE2 decoy for both neutralization of SARS-CoV-2 variants and killing of infected cells
- PMID: 37383226
- PMCID: PMC10293748
- DOI: 10.3389/fimmu.2023.1204543
A novel ACE2 decoy for both neutralization of SARS-CoV-2 variants and killing of infected cells
Abstract
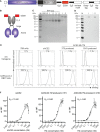
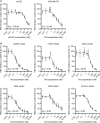
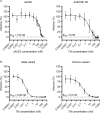
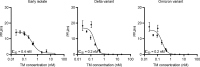
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to millions of infections and deaths worldwide. As this virus evolves rapidly, there is a high need for treatment options that can win the race against new emerging variants of concern. Here, we describe a novel immunotherapeutic drug based on the SARS-CoV-2 entry receptor ACE2 and provide experimental evidence that it cannot only be used for (i) neutralization of SARS-CoV-2 in vitro and in SARS-CoV-2-infected animal models but also for (ii) clearance of virus-infected cells. For the latter purpose, we equipped the ACE2 decoy with an epitope tag. Thereby, we converted it to an adapter molecule, which we successfully applied in the modular platforms UniMAB and UniCAR for retargeting of either unmodified or universal chimeric antigen receptor-modified immune effector cells. Our results pave the way for a clinical application of this novel ACE2 decoy, which will clearly improve COVID-19 treatment.
Keywords: ACE2 decoy; COVID-19; SARS–CoV–2; T-cell based immunotherapy; adapter CAR platform; bispecific antibody.
Copyright © 2023 Kegler, Drewitz, Arndt, Daglar, Rodrigues Loureiro, Mitwasi, Neuber, González Soto, Bartsch, Baraban, Ziehr, Heine, Nieter, Moreira-Soto, Kühne, Drexler, Seliger, Laube, Máthé, Pályi, Hajdrik, Forgách, Kis, Szigeti, Bergmann, Feldmann and Bachmann.
Conflict of interest statement
Author DM was employed by CROmed Translational Research Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- WHO . WHO COVID-19 dashboard (2023). Available at: https://covid19.who.int/ (Accessed May 04, 2023).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

