Randomized controlled phase IIa clinical trial of safety, pharmacokinetics and pharmacodynamics of tenofovir and tenofovir plus levonorgestrel releasing intravaginal rings used by women in Kenya
- PMID: 37383290
- PMCID: PMC10293630
- DOI: 10.3389/frph.2023.1118030
Randomized controlled phase IIa clinical trial of safety, pharmacokinetics and pharmacodynamics of tenofovir and tenofovir plus levonorgestrel releasing intravaginal rings used by women in Kenya
Abstract
Introduction: Globally, many young women face the overlapping burden of HIV infection and unintended pregnancy. Protection against both may benefit from safe and effective multipurpose prevention technologies.
Methods: Healthy women ages 18-34 years, not pregnant, seronegative for HIV and hepatitis B surface antigen, not using hormonal contraception, and at low risk for HIV were randomized 2:2:1 to continuous use of a tenofovir/levonorgestrel (TFV/LNG), TFV, or placebo intravaginal ring (IVR). In addition to assessing genital and systemic safety, we determined TFV concentrations in plasma and cervicovaginal fluid (CVF) and LNG levels in serum using tandem liquid chromatography-mass spectrometry. We further evaluated TFV pharmacodynamics (PD) through ex vivo CVF activity against both human immunodeficiency virus (HIV)-1 and herpes simplex virus (HSV)-2, and LNG PD using cervical mucus quality markers and serum progesterone for ovulation inhibition.
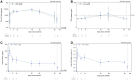
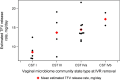
Results: Among 312 women screened, 27 were randomized to use one of the following IVRs: TFV/LNG (n = 11); TFV-only (n = 11); or placebo (n = 5). Most screening failures were due to vaginal infections. The median days of IVR use was 68 [interquartile range (IQR), 36-90]. Adverse events (AEs) were distributed similarly among the three arms. There were two non-product related AEs graded >2. No visible genital lesions were observed. Steady state geometric mean amount (ssGMA) of vaginal TFV was comparable in the TFV/LNG and TFV IVR groups, 43,988 ng/swab (95% CI, 31,232, 61,954) and 30337 ng/swab (95% CI, 18,152, 50,702), respectively. Plasma TFV steady state geometric mean concentration (ssGMC) was <10 ng/ml for both TFV IVRs. In vitro, CVF anti-HIV-1 activity showed increased HIV inhibition over baseline following TFV-eluting IVR use, from a median of 7.1% to 84.4% in TFV/LNG, 15.0% to 89.5% in TFV-only, and -27.1% to -20.1% in placebo participants. Similarly, anti-HSV-2 activity in CVF increased >50 fold after use of TFV-containing IVRs. LNG serum ssGMC was 241 pg/ml (95% CI 185, 314) with rapid rise after TFV/LNG IVR insertion and decline 24-hours post-removal (586 pg/ml [95% CI 473, 726] and 87 pg/ml [95% CI 64, 119], respectively).
Conclusion: TFV/LNG and TFV-only IVRs were safe and well tolerated among Kenyan women. Pharmacokinetics and markers of protection against HIV-1, HSV-2, and unintended pregnancy suggest the potential for clinical efficacy of the multipurpose TFV/LNG IVR.
Clinical trial registration: NCT03762382 [https://clinicaltrials.gov/ct2/show/NCT03762382].
Keywords: Africa; HIV; HSV-2; intravaginal ring; levonorgestrel; multipurpose technology; tenofovir.
© 2023 Mugo, Mudhune, Heffon, Thomas, McLellan-Lemal, Njoroge, Peacock, O’Connor, Nyagol, Ouma, Ridzon, Wiener, Isoherranen, Erikson, Ouattara, Yousefieh, Jacot, Haaland, Morrison, Haugen, Thurman, Allen, Baeten, Samandari and Doncel.
Conflict of interest statement
Jared M Baeten is an employee of Gilead Sciences, outside of the present work. Nina Isoherrranen has consultancy agreements with Boehringer-Ingelheim, Johnson and Johnson and Xenon Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- UNAIDS. 2017 Estimates from the Aids Info Online Database. In: UNAIDS, editor.
-
- UNAIDS. Unaids Fact Sheet Report on the Global Aids Epidemic (2020).
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

