Homocysteine-a retrospective and prospective appraisal
- PMID: 37384104
- PMCID: PMC10294675
- DOI: 10.3389/fnut.2023.1179807
Homocysteine-a retrospective and prospective appraisal
Abstract
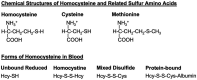
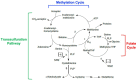
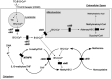
The biologically important amino acid homocysteine links sulfur, methionine, and one-carbon metabolism. This review describes its initial discovery, the identification of the clinical condition of "homocystinuria" and the recognition of its close relationship to folate and vitamin B12 metabolism. It discusses the history behind its current association with diverse diseases including neural tube defects, cardio- and cerebrovascular disease and, more recently, dementia and Alzheimer's Disease. It also explores current controversies and considers potential future research directions. It is intended to give a general overview of homocysteine in relation to health and disease.
Keywords: Alzheimer’s disease; dementia; folate; homocysteine; inborn errors of metabolism; neural tube defects; vascular disease; vitamin B12.
Copyright © 2023 McCaddon and Miller.
Conflict of interest statement
AM is a shareholder and Scientific Advisor for COBALZ Limited, a private Limited Company developing novel B-vitamin and antioxidant supplements. JM receives compensation as Associate Editor for the journal Nutrition Reviews, and has received within the last 3 years consulting compensation from Church and Dwight, Inc., a producer and seller of consumer goods including vitamin supplements. The study received funding from COBALZ Limited. The funder had the following involvement: APC costs.
Figures
References
-
- Miller JW. Homocysteine In: Caballero B, editor. Encyclopedia of Human Nutrition. Waltham, MA: Academic Press; (2013). 424–30.
-
- Butz L, du Vigneaud V. The formation of a homologue of cystine by the decompensation of methionine with sulfuric acid. J Biol Chem. (1932) 99:135–42. doi: 10.1016/S0021-9258(18)76074-2 - DOI
-
- Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. New York: McGraw Hill Inc; (1995).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

