Long non-coding RNAs modulate tumor microenvironment to promote metastasis: novel avenue for therapeutic intervention
- PMID: 37384249
- PMCID: PMC10299194
- DOI: 10.3389/fcell.2023.1164301
Long non-coding RNAs modulate tumor microenvironment to promote metastasis: novel avenue for therapeutic intervention
Abstract
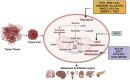
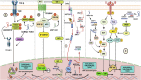
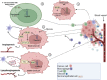
Cancer is a devastating disease and the primary cause of morbidity and mortality worldwide, with cancer metastasis responsible for 90% of cancer-related deaths. Cancer metastasis is a multistep process characterized by spreading of cancer cells from the primary tumor and acquiring molecular and phenotypic changes that enable them to expand and colonize in distant organs. Despite recent advancements, the underlying molecular mechanism(s) of cancer metastasis is limited and requires further exploration. In addition to genetic alterations, epigenetic changes have been demonstrated to play an important role in the development of cancer metastasis. Long non-coding RNAs (lncRNAs) are considered one of the most critical epigenetic regulators. By regulating signaling pathways and acting as decoys, guides, and scaffolds, they modulate key molecules in every step of cancer metastasis such as dissemination of carcinoma cells, intravascular transit, and metastatic colonization. Gaining a good knowledge of the detailed molecular basis underlying lncRNAs regulating cancer metastasis may provide previously unknown therapeutic and diagnostic lncRNAs for patients with metastatic disease. In this review, we concentrate on the molecular mechanisms underlying lncRNAs in the regulation of cancer metastasis, the cross-talk with metabolic reprogramming, modulating cancer cell anoikis resistance, influencing metastatic microenvironment, and the interaction with pre-metastatic niche formation. In addition, we also discuss the clinical utility and therapeutic potential of lncRNAs for cancer treatment. Finally, we also represent areas for future research in this rapidly developing field.
Keywords: anoikis resistance; cancer; immune modulation; long non-coding RNAs; metabolic reprogramming; metastasis; tumor microenvironment.
Copyright © 2023 Baba, Baba, Mir, Elfaki, Algehainy, Ullah, Barnawi, Altemani, Alanazi, Mustafa, Masoodi, Akil, Bhat and Macha.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Long non-coding RNAs and cancer metastasis: Molecular basis and therapeutic implications.Biochim Biophys Acta Rev Cancer. 2021 Apr;1875(2):188519. doi: 10.1016/j.bbcan.2021.188519. Epub 2021 Feb 4. Biochim Biophys Acta Rev Cancer. 2021. PMID: 33548345 Review.
-
Long noncoding RNAs in cancer metastasis.Nat Rev Cancer. 2021 Jul;21(7):446-460. doi: 10.1038/s41568-021-00353-1. Epub 2021 May 5. Nat Rev Cancer. 2021. PMID: 33953369 Free PMC article. Review.
-
Non-Coding RNAs Operate in the Crosstalk Between Cancer Metabolic Reprogramming and Metastasis.Front Oncol. 2020 May 29;10:810. doi: 10.3389/fonc.2020.00810. eCollection 2020. Front Oncol. 2020. PMID: 32547948 Free PMC article. Review.
-
Roles of lncRNAs in cancer: Focusing on angiogenesis.Life Sci. 2020 Jul 1;252:117647. doi: 10.1016/j.lfs.2020.117647. Epub 2020 Apr 8. Life Sci. 2020. PMID: 32275935 Review.
-
Roles of Non-Coding RNAs in Cervical Cancer Metastasis.Front Oncol. 2021 Mar 11;11:646192. doi: 10.3389/fonc.2021.646192. eCollection 2021. Front Oncol. 2021. PMID: 33777808 Free PMC article. Review.
Cited by
-
lncRNAs as prognostic markers and therapeutic targets in cuproptosis-mediated cancer.Clin Exp Med. 2024 Sep 26;24(1):226. doi: 10.1007/s10238-024-01491-0. Clin Exp Med. 2024. PMID: 39325172 Free PMC article. Review.
-
Exploring RNA cargo in extracellular vesicles for pleural mesothelioma detection.BMC Cancer. 2025 Feb 7;25(1):212. doi: 10.1186/s12885-025-13617-y. BMC Cancer. 2025. PMID: 39920655 Free PMC article.
-
Small protein DDX11-AS1-ORF encoded by lncRNA DDX11-AS1 promotes colorectal cancer progression through VEGFA-activated p38-MAPK pathway.Am J Cancer Res. 2025 Apr 15;15(4):1662-1672. doi: 10.62347/VRJE7714. eCollection 2025. Am J Cancer Res. 2025. PMID: 40371138 Free PMC article.
-
RNA splicing variants of the novel long non-coding RNA, CyKILR, possess divergent biological functions in non-small cell lung cancer.Mol Ther Nucleic Acids. 2024 Dec 5;36(1):102412. doi: 10.1016/j.omtn.2024.102412. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39807365 Free PMC article.
-
Role of the tumor microenvironment in cancer therapy: unveiling new targets to overcome drug resistance.Med Oncol. 2025 May 7;42(6):202. doi: 10.1007/s12032-025-02754-w. Med Oncol. 2025. PMID: 40332723 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

