Modulation of the thalamus by microburst vagus nerve stimulation: a feasibility study protocol
- PMID: 37384278
- PMCID: PMC10299807
- DOI: 10.3389/fneur.2023.1169161
Modulation of the thalamus by microburst vagus nerve stimulation: a feasibility study protocol
Abstract
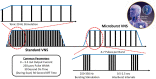
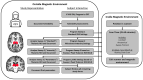
Vagus nerve stimulation (VNS) was the first device-based therapy for epilepsy, having launched in 1994 in Europe and 1997 in the United States. Since then, significant advances in the understanding of the mechanism of action of VNS and the central neurocircuitry that VNS modulates have impacted how the therapy is practically implemented. However, there has been little change to VNS stimulation parameters since the late 1990s. Short bursts of high frequency stimulation have been of increasing interest to other neuromodulation targets e.g., the spine, and these high frequency bursts elicit unique effects in the central nervous system, especially when applied to the vagus nerve. In the current study, we describe a protocol design that is aimed to assess the impact of high frequency bursts of stimulation, called "Microburst VNS", in subjects with refractory focal and generalized epilepsies treated with this novel stimulation pattern in addition to standard anti-seizure medications. This protocol also employed an investigational, fMRI-guided titration protocol that permits personalized dosing of Microburst VNS among the treated population depending on the thalamic blood-oxygen-level-dependent signal. The study was registered on clinicaltrials.gov (NCT03446664). The first subject was enrolled in 2018 and the final results are expected in 2023.
Keywords: drug-resistant epilepsy; feasibility study; focal epilepsy; generalized epilepsy; vagus nerve stimulation.
Copyright © 2023 Verner, Szaflarski, Allendorfer, Vonck, Giannicola and Microburst Study Group.
Conflict of interest statement
RV and GG are employees of LivaNova PLC or a subsidiary, and own stock and/or stock options with the sponsor of this study. KV was an investigator on the Microburst Feasibility Study. JS and JA developed the imaging protocol for this study under a consulting agreement with LivaNova USA, Inc. KV, JA, and JS have active consulting agreements with LivaNova PLC or its subsidiary businesses, related to advisory services, speaking services, and/or research activities. The Microburst Study Group consists of site investigators from each clinical study site. These investigators received some funding from LivaNova USA, Inc. to execute the Microburst Feasibility Study. No author was compensated for time spent writing this manuscript, and the content reflects the views of the authors and not LivaNova PLC or a subsidiary.
Figures

References
-
- Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. . Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. (2014) 55:432–41. 10.1111/epi.12534 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

