Clonal evolution in tyrosine kinase inhibitor-resistance: lessons from in vitro-models
- PMID: 37384296
- PMCID: PMC10294234
- DOI: 10.3389/fonc.2023.1200897
Clonal evolution in tyrosine kinase inhibitor-resistance: lessons from in vitro-models
Abstract
Introduction: Resistance in anti-cancer treatment is a result of clonal evolution and clonal selection. In chronic myeloid leukemia (CML), the hematopoietic neoplasm is predominantly caused by the formation of the BCR::ABL1 kinase. Evidently, treatment with tyrosine kinase inhibitors (TKIs) is tremendously successful. It has become the role model of targeted therapy. However, therapy resistance to TKIs leads to loss of molecular remission in about 25% of CML patients being partially due to BCR::ABL1 kinase mutations, while for the remaining cases, various other mechanisms are discussed.
Methods: Here, we established an in vitro-TKI resistance model against the TKIs imatinib and nilotinib and performed exome sequencing.
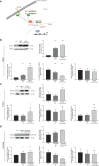
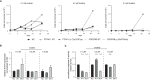
Results: In this model, acquired sequence variants in NRAS, KRAS, PTPN11, and PDGFRB were identified in TKI resistance. The well-known pathogenic NRAS p.(Gln61Lys) variant provided a strong benefit for CML cells under TKI exposure visible by increased cell number (6.2-fold, p < 0.001) and decreased apoptosis (-25%, p < 0.001), proving the functionality of our approach. The transfection of PTPN11 p.(Tyr279Cys) led to increased cell number (1.7-fold, p = 0.03) and proliferation (2.0-fold, p < 0.001) under imatinib treatment.
Discussion: Our data demonstrate that our in vitro-model can be used to study the effect of specific variants on TKI resistance and to identify new driver mutations and genes playing a role in TKI resistance. The established pipeline can be used to study candidates acquired in TKI-resistant patients, thereby providing new options for the development of new therapy strategies to overcome resistance.
Keywords: KRAS; NRAS; PDGFRB; PTPN11; chronic myeloid leukemia; drug resistance; imatinib; nilotinib.
Copyright © 2023 Kaehler, Osteresch, Künstner, Vieth, Esser, Möller, Busch, Vater, Spielmann, Cascorbi and Nagel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

