Reduction of nemo-like kinase increases lysosome biogenesis and ameliorates TDP-43-related neurodegeneration
- PMID: 37384409
- PMCID: PMC10425213
- DOI: 10.1172/JCI138207
Reduction of nemo-like kinase increases lysosome biogenesis and ameliorates TDP-43-related neurodegeneration
Abstract
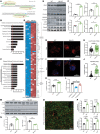
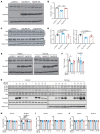
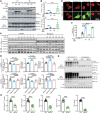
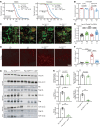
Protein aggregation is a hallmark of many neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS). Although mutations in TARDBP, encoding transactive response DNA-binding protein 43 kDa (TDP-43), account for less than 1% of all ALS cases, TDP-43-positive aggregates are present in nearly all ALS patients, including patients with sporadic ALS (sALS) or carrying other familial ALS-causing (fALS-causing) mutations. Interestingly, TDP-43 inclusions are also present in subsets of patients with frontotemporal dementia, Alzheimer's disease, and Parkinson's disease; therefore, methods of activating intracellular protein quality control machinery capable of clearing toxic cytoplasmic TDP-43 species may alleviate disease-related phenotypes. Here, we identify a function of nemo-like kinase (Nlk) as a negative regulator of lysosome biogenesis. Genetic or pharmacological reduction of Nlk increased lysosome formation and improved clearance of aggregated TDP-43. Furthermore, Nlk reduction ameliorated pathological, behavioral, and life span deficits in 2 distinct mouse models of TDP-43 proteinopathy. Because many toxic proteins can be cleared through the autophagy/lysosome pathway, targeted reduction of Nlk represents a potential approach to therapy development for multiple neurodegenerative disorders.
Keywords: Genetics; Lysosomes; Mouse models; Neurodegeneration; Neuroscience.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

