The ribosome-inactivating proteins MAP30 and Momordin inhibit SARS-CoV-2
- PMID: 37384752
- PMCID: PMC10310010
- DOI: 10.1371/journal.pone.0286370
The ribosome-inactivating proteins MAP30 and Momordin inhibit SARS-CoV-2
Abstract
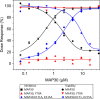
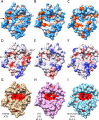
The continuing emergence of SARS-CoV-2 variants has highlighted the need to identify additional points for viral inhibition. Ribosome inactivating proteins (RIPs), such as MAP30 and Momordin which are derived from bitter melon (Momordica charantia), have been found to inhibit a broad range of viruses. MAP30 has been shown to potently inhibit HIV-1 with minimal cytotoxicity. Here we show that MAP30 and Momordin potently inhibit SARS-CoV-2 replication in A549 human lung cells (IC50 ~ 0.2 μM) with little concomitant cytotoxicity (CC50 ~ 2 μM). Both viral inhibition and cytotoxicity remain unaltered by appending a C-terminal Tat cell-penetration peptide to either protein. Mutation of tyrosine 70, a key residue in the active site of MAP30, to alanine completely abrogates both viral inhibition and cytotoxicity, indicating the involvement of its RNA N-glycosylase activity. Mutation of lysine 171 and lysine 215, residues corresponding to those in Ricin which when mutated prevented ribosome binding and inactivation, to alanine in MAP30 decreased cytotoxicity (CC50 ~ 10 μM) but also the viral inhibition (IC50 ~ 1 μM). Unlike with HIV-1, neither Dexamethasone nor Indomethacin exhibited synergy with MAP30 in the inhibition of SARS-CoV-2. From a structural comparison of the two proteins, one can explain their similar activities despite differences in both their active-sites and ribosome-binding regions. We also note points on the viral genome for potential inhibition by these proteins.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

