Dynamic mapping of proteome trafficking within and between living cells by TransitID
- PMID: 37385249
- PMCID: PMC10527209
- DOI: 10.1016/j.cell.2023.05.044
Dynamic mapping of proteome trafficking within and between living cells by TransitID
Abstract
The ability to map trafficking for thousands of endogenous proteins at once in living cells would reveal biology currently invisible to both microscopy and mass spectrometry. Here, we report TransitID, a method for unbiased mapping of endogenous proteome trafficking with nanometer spatial resolution in living cells. Two proximity labeling (PL) enzymes, TurboID and APEX, are targeted to source and destination compartments, and PL with each enzyme is performed in tandem via sequential addition of their small-molecule substrates. Mass spectrometry identifies the proteins tagged by both enzymes. Using TransitID, we mapped proteome trafficking between cytosol and mitochondria, cytosol and nucleus, and nucleolus and stress granules (SGs), uncovering a role for SGs in protecting the transcription factor JUN from oxidative stress. TransitID also identifies proteins that signal intercellularly between macrophages and cancer cells. TransitID offers a powerful approach for distinguishing protein populations based on compartment or cell type of origin.
Keywords: JUN; intercellular signaling; membraneless organelles; protein trafficking; proximity labeling; spatial proteomics; stress granules; tumor-associated macrophages.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.Y.T. is a scientific advisor to Third Rock Ventures and Nereid Therapeutics.
Figures
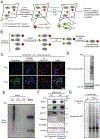
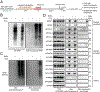





Update of
-
Dynamic mapping of proteome trafficking within and between living cells by TransitID.bioRxiv [Preprint]. 2023 Feb 8:2023.02.07.527548. doi: 10.1101/2023.02.07.527548. bioRxiv. 2023. Update in: Cell. 2023 Jul 20;186(15):3307-3324.e30. doi: 10.1016/j.cell.2023.05.044. PMID: 36798302 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

