Influence of HIV-1 Genomic RNA on the Formation of Gag Biomolecular Condensates
- PMID: 37385580
- PMCID: PMC10838171
- DOI: 10.1016/j.jmb.2023.168190
Influence of HIV-1 Genomic RNA on the Formation of Gag Biomolecular Condensates
Abstract
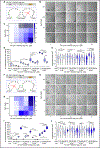
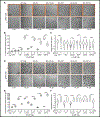
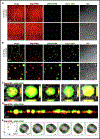
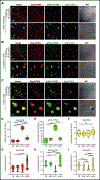
Biomolecular condensates (BMCs) play an important role in the replication of a growing number of viruses, but many important mechanistic details remain to be elucidated. Previously, we demonstrated that the pan-retroviral nucleocapsid (NC) and HIV-1 pr55Gag (Gag) proteins phase separate into condensates, and that HIV-1 protease (PR)-mediated maturation of Gag and Gag-Pol precursor proteins yields self-assembling BMCs that have HIV-1 core architecture. Using biochemical and imaging techniques, we aimed to further characterize the phase separation of HIV-1 Gag by determining which of its intrinsically disordered regions (IDRs) influence the formation of BMCs, and how the HIV-1 viral genomic RNA (gRNA) could influence BMC abundance and size. We found that mutations in the Gag matrix (MA) domain or the NC zinc finger motifs altered condensate number and size in a salt-dependent manner. Gag BMCs were also bimodally influenced by the gRNA, with a condensate-promoting regime at lower protein concentrations and a gel dissolution at higher protein concentrations. Interestingly, incubation of Gag with CD4+ T cell nuclear lysates led to the formation of larger BMCs compared to much smaller ones observed in the presence of cytoplasmic lysates. These findings suggest that the composition and properties of Gag-containing BMCs may be altered by differential association of host factors in nuclear and cytosolic compartments during virus assembly. This study significantly advances our understanding of HIV-1 Gag BMC formation and provides a foundation for future therapeutic targeting of virion assembly.
Keywords: biomolecular condensates; gag polyprotein; human immunodeficiency virus-type 1; phase diagrams; viral genomic RNA.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Update of
-
Influence of HIV-1 genomic RNA on the formation of Gag biomolecular condensates.bioRxiv [Preprint]. 2023 Feb 23:2023.02.23.529585. doi: 10.1101/2023.02.23.529585. bioRxiv. 2023. Update in: J Mol Biol. 2023 Aug 15;435(16):168190. doi: 10.1016/j.jmb.2023.168190. PMID: 36865181 Free PMC article. Updated. Preprint.
References
-
- Alberti S, Hyman AA, (2021). Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213. - PubMed
-
- Pessina F, Gioia U, Brandi O, Farina S, Ceccon M, Francia S, et al., (2021). DNA Damage Triggers a New Phase in Neurodegeneration Trends in Genetics. Apr 37 (4), 337–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

