Antiviral CD8+ T-cell immune responses are impaired by cigarette smoke and in COPD
- PMID: 37385655
- PMCID: PMC10397470
- DOI: 10.1183/13993003.01374-2022
Antiviral CD8+ T-cell immune responses are impaired by cigarette smoke and in COPD
Abstract
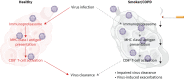
Background: Virus infections drive COPD exacerbations and progression. Antiviral immunity centres on the activation of virus-specific CD8+ T-cells by viral epitopes presented on major histocompatibility complex (MHC) class I molecules of infected cells. These epitopes are generated by the immunoproteasome, a specialised intracellular protein degradation machine, which is induced by antiviral cytokines in infected cells.
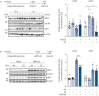
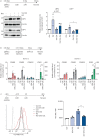
Methods: We analysed the effects of cigarette smoke on cytokine- and virus-mediated induction of the immunoproteasome in vitro, ex vivo and in vivo using RNA and Western blot analyses. CD8+ T-cell activation was determined in co-culture assays with cigarette smoke-exposed influenza A virus (IAV)-infected cells. Mass-spectrometry-based analysis of MHC class I-bound peptides uncovered the effects of cigarette smoke on inflammatory antigen presentation in lung cells. IAV-specific CD8+ T-cell numbers were determined in patients' peripheral blood using tetramer technology.
Results: Cigarette smoke impaired the induction of the immunoproteasome by cytokine signalling and viral infection in lung cells in vitro, ex vivo and in vivo. In addition, cigarette smoke altered the peptide repertoire of antigens presented on MHC class I molecules under inflammatory conditions. Importantly, MHC class I-mediated activation of IAV-specific CD8+ T-cells was dampened by cigarette smoke. COPD patients exhibited reduced numbers of circulating IAV-specific CD8+ T-cells compared to healthy controls and asthmatics.
Conclusion: Our data indicate that cigarette smoke interferes with MHC class I antigen generation and presentation and thereby contributes to impaired activation of CD8+ T-cells upon virus infection. This adds important mechanistic insight on how cigarette smoke mediates increased susceptibility of smokers and COPD patients to viral infections.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of Interest: H. Ma reports support for the present manuscript from the German Center for Lung Research (DZL). M. Nakayama reports overseas grant from Uehara Memorial Foundation (Japan) and overseas grant from Shiga university of Medical Science, outside the submitted work. A.L. Mora reports support for the present manuscript from NIH (NIH U01 HL1455550-01 and NIH NHLBI R01 HL149825). J.S. Lee reports participation on clinical adjudication committee with Janssen R&D, outside the submitted work. S. Krauss-Etschmann reports support for the present manuscript from the German Center for Lung Research. K. Milger reports consulting fees and lecture honoraria from AstraZeneca, GSK, Janssen, Novartis and Sanofi, outside the submitted work. C.A. Staab-Weijnitz reports support for the present manuscript from Helmholtz Association, German Center for Lung Research (DZL) and Deutsche Forschungsgemeinschaft (DFG) within the Research Training Group GRK2338. K.I. Gaede reports support for the present manuscript from Research Center Borstel – Leibniz Lung Center – BioMaterialBank North, Airway Research Center North, German Center for Lung Research (DZL), PopGen 2.0 Network (P2N). K.I. Gaede also holds a leadership role as member of the Board of Directors of the TMF (www.tmf-ev.de), outside the submitted work. I.E. Kammerl reports support for the present manuscript from ERS (Short Term Fellowship). All other authors have no potential conflicts of interest to declare.
Figures




Comment in
-
Blunted adaptive immune responses and acute exacerbations of COPD: breaking the code.Eur Respir J. 2023 Aug 3;62(2):2301030. doi: 10.1183/13993003.01030-2023. Print 2023 Aug. Eur Respir J. 2023. PMID: 37536726 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
