Modifiers of COVID-19 vaccine efficacy: Results from four COVID-19 prevention network efficacy trials
- PMID: 37385888
- PMCID: PMC10288314
- DOI: 10.1016/j.vaccine.2023.06.066
Modifiers of COVID-19 vaccine efficacy: Results from four COVID-19 prevention network efficacy trials
Abstract
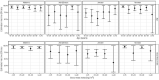
Questions remain regarding the effect of baseline host and exposure factors on vaccine efficacy (VE) across pathogens and vaccine platforms. We report placebo-controlled data from four Phase 3 COVID-19 trials during the early period of the pandemic. This was a cross-protocol analysis of four randomized, placebo-controlled efficacy trials (Moderna/mRNA1273, AstraZeneca/AZD1222, Janssen/Ad26.COV2.S, and Novavax/NVX-CoV2373) using a harmonized design. Trials were conducted in the United States and international sites in adults ≥ 18 years of age. VE was assessed for symptomatic and severe COVID-19. We analyzed 114,480 participants from both placebo and vaccine arms, enrolled July 2020 to February 2021, with follow up through July 2021. VE against symptomatic COVID-19 showed little heterogeneity across baseline socio-demographic, clinical or exposure characteristics, in either univariate or multivariate analysis, regardless of vaccine platform. Similarly, VE against severe COVID-19 in the single trial (Janssen) with sufficient endpoints for analysis showed little evidence of heterogeneity. COVID-19 VE is not influenced by baseline host or exposure characteristics across efficacy trials of different vaccine platforms and countries when well matched to circulating virus strains. This supports use of these vaccines, regardless of platform type, as effective tools in the near term for reducing symptomatic and severe COVID-19, particularly for older individuals and those with common co-morbidities during major variant shifts. Clinical trial registration numbers: NCT04470427, NCT04516746, NCT04505722, and NCT04611802.
Keywords: Comorbidity; Effect modifier; Environmental exposure; Epidemiologic; Occupational exposure; SARS-CoV-2; Vaccine efficacy.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Brett Leav reports a relationship with Moderna Inc that includes: employment. Jacqueline Miller reports a relationship with Moderna Inc that includes: employment. Kathryn Shoemaker reports a relationship with AstraZeneca R&D Gaithersburg that includes: employment and equity or stocks. An Vandenbosch reports a relationship with Janssen Pharmaceuticals Inc that includes: employment. Jerald Sadoff reports a relationship with Janssen Pharmaceuticals Inc that includes: employment. Wayne Woo reports a relationship with Novavax Inc that includes: employment. Iksung Cho reports a relationship with Novavax Inc that includes: employment. Lisa M. Dunkle reports a relationship with Novavax Inc that includes: employment. Peter Gilbert reports a relationship with Janssen Pharmaceuticals Inc that includes: consulting or advisory. Hana M. El Sahly reports a relationship with Gilead Sciences Inc that includes: funding grants. Hana M. El Sahly reports a relationship with Janssen Global Services LLC that includes: funding grants. Hana M. El Sahly reports a relationship with Merck & Co Inc that includes: funding grants. Hana M. El Sahly reports a relationship with Sanofi Pasteur Inc that includes: funding grants. Kathleen M. Neuzil reports a relationship with Pfizer Inc that includes: funding grants. Ann R. Falsey reports a relationship with Biofire Diagnostics Inc that includes: funding grants. Ann R. Falsey reports a relationship with Janssen Biotech Inc that includes: funding grants. Ann R. Falsey reports a relationship with Merck & Co Inc that includes: funding grants. Ann R. Falsey reports a relationship with Pfizer Inc that includes: funding grants. Ann R. Falsey reports a relationship with Novavax Inc that includes: consulting or advisory.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069476/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- S10 AI174104/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI148689/AI/NIAID NIH HHS/United States
- UM1 AI148450/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States

