Amyloid β-based therapy for Alzheimer's disease: challenges, successes and future
- PMID: 37386015
- PMCID: PMC10310781
- DOI: 10.1038/s41392-023-01484-7
Amyloid β-based therapy for Alzheimer's disease: challenges, successes and future
Abstract
Amyloid β protein (Aβ) is the main component of neuritic plaques in Alzheimer's disease (AD), and its accumulation has been considered as the molecular driver of Alzheimer's pathogenesis and progression. Aβ has been the prime target for the development of AD therapy. However, the repeated failures of Aβ-targeted clinical trials have cast considerable doubt on the amyloid cascade hypothesis and whether the development of Alzheimer's drug has followed the correct course. However, the recent successes of Aβ targeted trials have assuaged those doubts. In this review, we discussed the evolution of the amyloid cascade hypothesis over the last 30 years and summarized its application in Alzheimer's diagnosis and modification. In particular, we extensively discussed the pitfalls, promises and important unanswered questions regarding the current anti-Aβ therapy, as well as strategies for further study and development of more feasible Aβ-targeted approaches in the optimization of AD prevention and treatment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
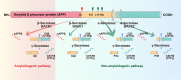
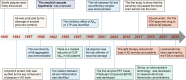

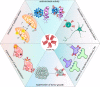
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

