Compressive forces stabilize microtubules in living cells
- PMID: 37386067
- PMCID: PMC10569437
- DOI: 10.1038/s41563-023-01578-1
Compressive forces stabilize microtubules in living cells
Abstract
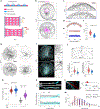
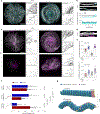
Microtubules are cytoskeleton components with unique mechanical and dynamic properties. They are rigid polymers that alternate phases of growth and shrinkage. Nonetheless, the cells can display a subset of stable microtubules, but it is unclear whether microtubule dynamics and mechanical properties are related. Recent in vitro studies suggest that microtubules have mechano-responsive properties, being able to stabilize their lattice by self-repair on physical damage. Here we study how microtubules respond to cycles of compressive forces in living cells and find that microtubules become distorted, less dynamic and more stable. This mechano-stabilization depends on CLASP2, which relocates from the end to the deformed shaft of microtubules. This process seems to be instrumental for cell migration in confined spaces. Overall, these results demonstrate that microtubules in living cells have mechano-responsive properties that allow them to resist and even counteract the forces to which they are subjected, being a central mediator of cellular mechano-responses.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

