Immunomodulatory response in an experimental model of brain death
- PMID: 37386074
- PMCID: PMC10310852
- DOI: 10.1038/s41598-023-36629-9
Immunomodulatory response in an experimental model of brain death
Abstract
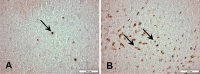
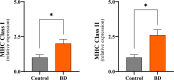
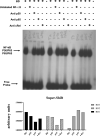
Liver transplantation has come a long way and is now regarded as the gold standard treatment for end-stage liver failure. The great majority of livers utilized in transplantation come from brain-dead donors. A broad inflammatory response characterizes BD, resulting in multiorgan damage. This process is primarily mediated by cytokines, which increase the immunogenicity of the graft. In male Lewis rats, we evaluated the immune response in a BD liver donor and compared it to that of a control group. We studied two groups: Control and BD (rats subjected to BD by increasing intracranial pressure). After the induction of BD, there was an intense rise in blood pressure followed by a fall. There were no significant differences observed between the groups. Blood tissue and hepatic tissue analyzes showed an increase in plasma concentrations of liver enzymes (AST, ALT, LDH and ALP), in addition to pro-inflammatory cytokines and macrophages in liver tissue in animals submitted to BD. The current study found that BD is a multifaceted process that elicits both a systemic immune response and a local inflammatory response in liver tissue. Our findings strongly suggested that the immunogenicity of plasma and liver increased with time following BD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Danobeitia JS. The brain-dead organ donor: A missed opportunity for early intervention. J. Transplant. Technol. Res. 2012;2:3. doi: 10.4172/2161-0991.1000e117. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

