Immunological imprinting of humoral immunity to SARS-CoV-2 in children
- PMID: 37386081
- PMCID: PMC10310754
- DOI: 10.1038/s41467-023-39575-2
Immunological imprinting of humoral immunity to SARS-CoV-2 in children
Abstract
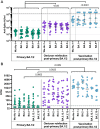
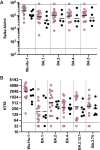
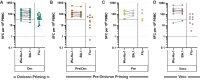
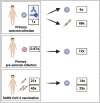
Omicron variants of SARS-CoV-2 are globally dominant and infection rates are very high in children. We measure immune responses following Omicron BA.1/2 infection in children aged 6-14 years and relate this to prior and subsequent SARS-CoV-2 infection or vaccination. Primary Omicron infection elicits a weak antibody response with poor functional neutralizing antibodies. Subsequent Omicron reinfection or COVID-19 vaccination elicits increased antibody titres with broad neutralisation of Omicron subvariants. Prior pre-Omicron SARS-CoV-2 virus infection or vaccination primes for robust antibody responses following Omicron infection but these remain primarily focussed against ancestral variants. Primary Omicron infection thus elicits a weak antibody response in children which is boosted after reinfection or vaccination. Cellular responses are robust and broadly equivalent in all groups, providing protection against severe disease irrespective of SARS-CoV-2 variant. Immunological imprinting is likely to act as an important determinant of long-term humoral immunity, the future clinical importance of which is unknown.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

