Inhibition of DRP1-dependent mitochondrial fission by Mdivi-1 alleviates atherosclerosis through the modulation of M1 polarization
- PMID: 37386574
- PMCID: PMC10311781
- DOI: 10.1186/s12967-023-04270-9
Inhibition of DRP1-dependent mitochondrial fission by Mdivi-1 alleviates atherosclerosis through the modulation of M1 polarization
Abstract
Background: Inflammation and immune dysfunction with classically activated macrophages(M1) infiltration are important mechanisms in the progression of atherosclerosis (AS). Dynamin-related protein 1 (DRP1)-dependent mitochondrial fission is a novel target for alleviating inflammatory diseases. This study aimed to investigate the effects of DRP1 inhibitor Mdivi-1 on AS.
Methods: ApoE-/- mice were fed with a high-fat diet supplemented with or without Mdivi-1. RAW264.7 cells were stimulated by ox-LDL, pretreated with or without MCC950, Mito-TEMPO, or Mdivi-1. The burden of plaques and foam cell formation were determined using ORO staining. The blood lipid profles and inflammatory cytokines in serum were detected by commercial kits and ELISA, respectively. The mRNA expression of macrophage polarization markers, activation of NLRP3 and the phosphorylation state of DRP1 were detected. Mitochondrial reactive oxygen species (mito-ROS), mitochondrial staining, ATP level and mitochondrial membrane potential were detected by mito-SOX, MitoTracker, ATP determination kit and JC-1 staining, respectively.
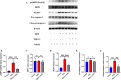
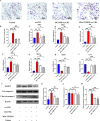
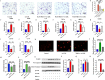
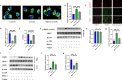
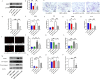
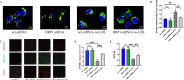
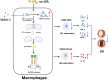
Results: In vivo, Mdivi-1 reduced the plaque areas, M1 polarization, NLRP3 activation and DRP1 phosphorylation at Ser616. In vitro, oxidized low-density lipoprotein (ox-LDL) triggered M1 polarization, NLRP3 activation and abnormal accumulation of mito-ROS. MCC950 and Mito-TEMPO suppressed M1 polarization mediated foam cell formation. Mito-TEMPO significantly inhibited NLRP3 activation. In addition, Mdivi-1 reduced foam cells by inhibiting M1 polarization. The possible mechanisms responsible for the anti-atherosclerotic effects of Mdivi-1 on reducing M1 polarization were associated with suppressing mito-ROS/NLRP3 pathway by inhibiting DRP1 mediated mitochondrial fission. In vitro, similar results were observed by DRP1 knockdown.
Conclusion: Inhibition of DRP1-dependent mitochondrial fission by Mdivi-1 alleviated atherogenesis via suppressing mito-ROS/NLRP3-mediated M1 polarization, indicating DRP1-dependent mitochondrial fission as a potential therapeutic target for AS.
Keywords: Atherosclerosis; DRP1; M1 polarization; Mdivi-1; Mitochondrial fission; NLRP3 inflammasome.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential competing interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

