Chronic stability of a neuroprosthesis comprising multiple adjacent Utah arrays in monkeys
- PMID: 37386891
- PMCID: PMC7617000
- DOI: 10.1088/1741-2552/ace07e
Chronic stability of a neuroprosthesis comprising multiple adjacent Utah arrays in monkeys
Abstract
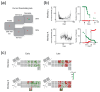
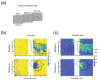
Objective. Electrical stimulation of visual cortex via a neuroprosthesis induces the perception of dots of light ('phosphenes'), potentially allowing recognition of simple shapes even after decades of blindness. However, restoration of functional vision requires large numbers of electrodes, and chronic, clinical implantation of intracortical electrodes in the visual cortex has only been achieved using devices of up to 96 channels. We evaluated the efficacy and stability of a 1024-channel neuroprosthesis system in non-human primates (NHPs) over more than 3 years to assess its suitability for long-term vision restoration.Approach.We implanted 16 microelectrode arrays (Utah arrays) consisting of 8 × 8 electrodes with iridium oxide tips in the primary visual cortex (V1) and visual area 4 (V4) of two sighted macaques. We monitored the animals' health and measured electrode impedances and neuronal signal quality by calculating signal-to-noise ratios of visually driven neuronal activity, peak-to-peak voltages of the waveforms of action potentials, and the number of channels with high-amplitude signals. We delivered cortical microstimulation and determined the minimum current that could be perceived, monitoring the number of channels that successfully yielded phosphenes. We also examined the influence of the implant on a visual task after 2-3 years of implantation and determined the integrity of the brain tissue with a histological analysis 3-3.5 years post-implantation.Main results. The monkeys remained healthy throughout the implantation period and the device retained its mechanical integrity and electrical conductivity. However, we observed decreasing signal quality with time, declining numbers of phosphene-evoking electrodes, decreases in electrode impedances, and impaired performance on a visual task at visual field locations corresponding to implanted cortical regions. Current thresholds increased with time in one of the two animals. The histological analysis revealed encapsulation of arrays and cortical degeneration. Scanning electron microscopy on one array revealed degradation of IrOxcoating and higher impedances for electrodes with broken tips.Significance. Long-term implantation of a high-channel-count device in NHP visual cortex was accompanied by deformation of cortical tissue and decreased stimulation efficacy and signal quality over time. We conclude that improvements in device biocompatibility and/or refinement of implantation techniques are needed before future clinical use is feasible.
Keywords: Utah arrays; V1; V4; blindness; microstimulation; neuroprosthesis; non-human primate.
Creative Commons Attribution license.
Conflict of interest statement
P R and X C are co-founders and shareholders of a neurotechnology start-up, Phosphoenix BV (Netherlands) (
Figures





References
-
- Teutsch SM, McCoy MA, Woodburyand RB, Welp A, editors. National Academies of Sciences, Engineering, and Medicine. Making Eye Health a Population Health Imperative: Vision for Tomorrow. National Academies Press; Washington, DC: 2016. - PubMed
-
- Roska B, Sahel J-A. Restoring vision. Nature. 2018;557:359–67. - PubMed
-
- Lewis PM, Rosenfeld JV. Electrical stimulation of the brain and the development of cortical visual prostheses: an historical perspective. Brain Res. 2016;1630:208–24. - PubMed
-
- Button J, Putnam T. Visual responses to cortical stimulation in the blind. J Iowa Med Soc. 1962;52:17–21.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
